Author Interviews, Macular Degeneration, Ophthalmology, PNAS / 27.10.2021
Fluoxetine – Prozac – May Be Beneficial for Dry Macular Degeneration
MedicalResearch.com Interview with:
Bradley D. Gelfand PhD
Center for Advanced Vision Science
Department of Ophthalmology
Department of Biomedical Engineering
University of Virginia School of Medicine
Charlottesville, VA 22908
MedicalResearch.com: What is the background for this study? Would you briefly describe dry AMD?
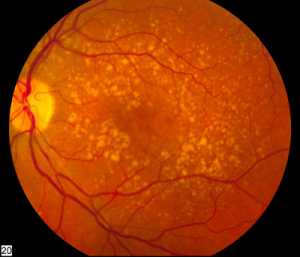
Response: Dry age-related macular degeneration (AMD) is a form of AMD that affects about 11 million people in the United States, and many millions more worldwide. Dry AMD is a disease affecting the macula, the central part of our retina that is responsible for fine visual acuity tasks - things like reading, driving, and recognizing faces. Dry AMD typically develops in people in their 6th, 7th, and 8th decades of life and begins with small changes to the retina that are unlikely to affect vision at first. As the disease progresses, it can develop into more advanced stages ("wet" AMD and geographic atrophy), which can cause blindness. Unfortunately, there is no approved treatment that can prevent dry AMD or its progression to advanced blinding stages.
(more…)