19 Nov Precise Structure of Cannabis Brain Receptor Defined
MedicalResearch.com Interview with:
Dan Rosenbaum, Ph.D.
Principal Investigator
Department of Biophysics
The University of Texas Southwestern Medical Center
Dallas, Texas
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: This study focuses on the structure of the human CB1 cannabinoid receptor.
The CB1 protein is a membrane-embedded G protein-coupled receptor (GPCR) in the brain and peripheral tissues that responds to a variety of different compounds, including endogenous lipid messengers (‘endocannabinoids’), plant natural products (such as THC from the Cannabis sativa plant i.e. marijuana), and synthetic antagonists (such as the taranabant ligand used for this study). The CB1 receptor is involved in regulating neurotransmission in vertebrates, and is a potential therapeutic target for numerous conditions including obesity, pain, and epilepsy.
The main findings of this study entailed the solution of the high-resolution crystal structure of human CB1 receptor bound to the inhibitor taranabant. This structure revealed the precise shape of the inhibitor binding pocket, which is also responsible for binding THC and endocannabinoids. In addition to helping explain the mechanism of inhibitor and THC binding, our structure provides a framework for computational studies of binding to a large diversity of cannabinoid modulators of therapeutic importance.
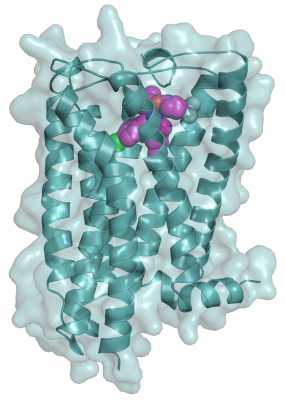
Illustration of human cannabinoid receptor (blue) with cannabinoid inhibitor taranabant (magenta) bound at receptor’s binding pocket sitting on molecular surface (gray)
UT Southwestern
MedicalResearch.com: What should readers take away from your report?
Response: Readers should take away from our study that we now have a precise atomic view of the site of action of different chemicals that stimulate or inhibit cannabinoid signaling. The study also illuminated several features of the protein that are important to its function, such as the involvement of the amino-terminal region (beginning of the protein) in cannabinoid binding and the presence of a membrane-embedded pathway for lipophilic modulators to enter into the receptor’s ligand binding pocket.
MedicalResearch.com: What recommendations do you have for future research as a result of this study?
Response: Future research will move in two directions.
First, we will use the structure to carry out computational docking studies of CB1 binding to different ligands, and complement these studies with biochemical experiments to validate the predictions. This work will help us to understand the molecular basis of affinity and selectivity for existing cannabinoid receptor modulators.
Second, we will use the tools and methods developed for this study as a basis for solving structures of CB1 in its agonist-bound (i.e. THC-bound) and active conformations.
MedicalResearch.com: Is there anything else you would like to add?
Response: While CB1 signaling has diverse functions in the brain and periphery, one of the major therapeutic areas that has been explored is treatment of obesity.
This application of CB1 inhibitors arises due to CB1’s involvement in regulation of energy metabolism. Currently CB1 inhibitors such as taranabant are not used in the clinic due to adverse psychotropic side effects. However future research will aim to avoid these side effects while maintaining efficacy in weight loss. Our structure of CB1 can be used to help understand how modifications of existing CB1 inhibitors (such as taranabant) will impact the affinity and selectivity of these substances.
MedicalResearch.com: Thank you for your contribution to the MedicalResearch.com community.
Note: Content is Not intended as medical advice. Please consult your health care provider regarding your specific medical condition and questions.
More Medical Research Interviews on MedicalResearch.com
[wysija_form id=”5″]
Last Updated on November 19, 2016 by Marie Benz MD FAAD

