06 Feb The Phenotypic Spectrum of a Mutation Hotspot Responsible for the Short QT Syndrome
MedicalResearch.com Interview with:
Dan Hu (Helen), MD. PhD. FAHA. FACC. FHRS.
Research Scientist II, Associate Professor
Clinical Consultant of Molecular Genetic Department
SCRO Chair of Stem Cell Center
Masonic Medical Research Laboratory
Utica, NY 13501
MedicalResearch.com: What is the background for this study?
Response: Short QT Syndrome (SQTS) is a rare genetic disease characterized by an abnormally short QT interval in subjects with structurally normal hearts. It is a recognized cause of cardiac rhythm disorders, including both atrial and ventricular arrhythmias, and sudden cardiac death (SCD). As an inherited channelopathy, the molecular basis for SQTS has been associated with mutations in 6 genes: KCNH2 (IKr, SQTS1), KCNQ1 (IKs, SQTS2), and KCNJ2 (IK1, SQTS3), which encode different potassium channels; CACNA1C, CACNB2b and CACNA2D1 (SQTS4-6), which encode the L-type calcium channel (ICa). This study sought to evaluate the phenotypic and functional expression of an apparent hotspot mutation associated with SQTS.
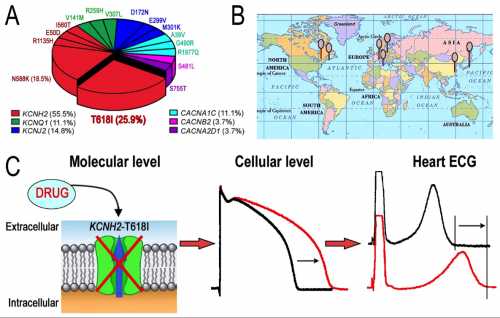
Distribution and Therapy of KCNH2-T618I Mutation in Short QT Syndrome. The proportion of KCNH2-T618I among genetic identified SQTS probands is the highest (25.9%, A), and the mutation is discovered in unrelated SQTS families from North America, Europe and Asia (B). KCNH2-T618I mutation leads to an increase in the repolarizing potassium current (IKr) and a decrease in action potential duration, which shortens the QT interval. With the IKr blocking effect by certain drugs, such as Quinidine, the action potential duration and QT/QTc get ameliorative, and VT/VF could be suppressed (C).
MedicalResearch.com: What are the main findings? What should readers take away from your report?
Response: We find that KCNH2-T618I is the highly frequent mutation associated with SQTS worldwide (18 members of 7 unrelated families; 10 males; Median age, 24.0 years), with a high incidence and family history of VT/VF or SCD, and complete penetrance after long term follow-up. But there is no atrial arrhythmias observed in any mutant carrier. It accounts for 25.9% of genetically identified SQTS probands without clear gender preference. ICD implantation is still the first-choice therapy, although the high rates of inappropriate shock and complication are also encountered. Quinidine is effective in prolonging QTc, but whether this translates into eliminated severe ventricular arrhythmias and decreased SCD is still unknown. Bepridil may be the new alternative to prevent VT/VF in SQTS. Functional studies with KCNE2 revealed a significant increase of IKr,tail-current density in homozygous (119.0%) and heterozygous (74.6%) expression compared with WT. AP clamp recordings showed IKr was larger and peak repolarizing current occurred earlier in mutant vs. WT channels. This major gain of function in IKr caused by mutation will lead to abbreviation of action potential and QT, giving rise to the substrate for the development of reentrant arrhythmias.
MedicalResearch.com: What recommendations do you have for future research as a result of this study?
Response: Although it is the largest collection (by absolute number) of SQTS carriers with same mutation thus far reported, the sample size of our study is necessarily small because of the rarity of the syndrome. Further long-term follow-up of patients are needed to clarify the prognosis of the mutation carriers and the most efficacious drug approaches to therapy. Stem cell model may also help to further identify the clinical characters and prognosis in the future.
MedicalResearch.com: Is there anything else you would like to add?
Response: Dr. Dan Hu is the main investigator in Masonic Medical Research Laboratory, Utica, NY. Her field involves translational and clinical cardiology, specifically in the area of electrophysiology and arrhythmia, such as inherited arrhythmogenic cardiac syndromes. She has contributed to discovery of 8 new genes related to Brugada and Early repolarization syndromes, also known as “J Wave Syndromes”. Besides that, she is also experienced on Long/Short QT syndrome, atrial fibrillation, WPW syndrome, sudden cardiac death, arrhythmias related to myocardial infarction and heart failure, and cardiomyopathy, et al. Her expertise also extends to cardiac pharmacology, therapeutics, stem cells, epidemiology, etc.
Her work has led to top peer review papers and highly cited. She has more than 70 publications, 70 abstracts, 3 books, and 70 invited lectures around the world. Now she is serving as Fellow of HRS (Heart Rhythm Society), Fellow of AHA (American Heart Association), Fellow of ACC (American College of Cardiology), and member of several well-known international societies, such as CES, APS. She is also the Vice President of Inherited Arrhythmias Committee in CHRS (Chinese Heart Rhythm Society). Dr. Hu has won several medical awards, including first place of YIA in ACC-NY chapter, and Gordon K. Moe YIA, et al. She has been invited to review grant application in USA and Netherland, and serve as reviewer or board member of prominent internationally circulated journals in the field, such as Circulation, Science – Translational Medicine, Heart Rhythm, Plos One, et al. Since 2016, she has been selected as image ambassador of HRS.
MedicalResearch.com: Any disclosures?
Nothing to disclose.
Citation:
Distribution and Therapy of KCNH2-T618I Mutation in Short QT Syndrome. The proportion of KCNH2-T618I among genetic identified SQTS probands is the highest (25.9%, A), and the mutation is discovered in unrelated SQTS families from North America, Europe and Asia (B). KCNH2-T618I mutation leads to an increase in the repolarizing potassium current (IKr) and a decrease in action potential duration, which shortens the QT interval. With the IKr blocking effect by certain drugs, such as Quinidine, the action potential duration and QT/QTc get ameliorative, and VT/VF could be suppressed (C).
Note: Content is Not intended as medical advice. Please consult your health care provider regarding your specific medical condition and questions.
More Medical Research Interviews on MedicalResearch.com
Last Updated on February 6, 2017 by Marie Benz MD FAAD

