01 Jan Transcranial Stimulation Has Potential as Add-On Therapy For Bipolar Depression
MedicalResearch.com Interview with:
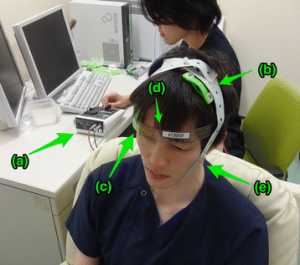
tDCS administration at National Center of Neurology and Psychiatry Hospital. A subject (front) sits on a sofa relaxed, and a researcher (behind) controls the tDCS device (a). In this picture, anodal (b) and cathodal (c) electrodes with 35-cm2 size are put on F3 and right supraorbital region, respectively. We use a head strap (d) for convenience and reproducibility, and also use a rubber band (e) for reducing resistance
Wikipedia file
Andre Russowsky Brunoni, MD, PhD
Coordinator, Service of Interdisciplinary Neuromodulation, Laboratory of Neurosciences Department and Institute of Psychiatry
Coordinator, Interdisciplinary Center for Applied Neuromodulation, University Hospital
University of São Paulo
São Paulo, Brasil
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: In this study, our aim was to evaluate the safety and efficacy of transcranial direct current stimulation (tDCS) as an add-on treatment for patients with bipolar depression. There are a only few treatment alternatives for bipolar depression, which often have important side effects. Thus, we wanted to evaluate the efficacy of this non-pharmacological treatment.
We found that active vs. sham tDCS effected greater response and remission for patients with bipolar depression. The frequency of adverse effects was similar, including treatment-emergent affective switches. However, higher rates of skin redness were observed in the active group.
MedicalResearch.com: What should clinicians and patients take away from your report?
Response: In this preliminary study, tDCS showed efficacy and safety for treating bipolar depression. It can be a potential add-on treatment for these patients.
MedicalResearch.com: What recommendations do you have for future research as a result of this study?
Response: The sample size was small and therefore our results were preliminary. They should be replicated in a larger sample. Also, the trial only lasted 6 weeks, so future research could also explore the effects of tDCS in the maintenance phase of bipolar depression.
MedicalResearch.com: Thank you for your contribution to the MedicalResearch.com community.
Citation:
Bernardo Sampaio-Junior, Gabriel Tortella, Lucas Borrione, Adriano H. Moffa, Rodrigo Machado-Vieira, Eric Cretaz, Adriano Fernandes da Silva, Renério Fraguas, Luana V. Aparício, Izio Klein, Beny Lafer, Stephan Goerigk, Isabela Martins Benseñor, Paulo Andrade Lotufo, Wagner F. Gattaz, André Russowsky Brunoni. Efficacy and Safety of Transcranial Direct Current Stimulation as an Add-on Treatment for Bipolar DepressionA Randomized Clinical Trial. JAMA Psychiatry. Published online December 27, 2017. doi:10.1001/jamapsychiatry.2017.4040
https://jamanetwork.com/journals/jamapsychiatry/article-abstract/2666768
Note: Content is Not intended as medical advice. Please consult your health care provider regarding your specific medical condition and questions.
[wysija_form id=”1″]
Last Updated on January 1, 2018 by Marie Benz MD FAAD
