
07 Oct Buprenorphine: New Views on an Old Drug

Leana Pande
MedicalResearch.com Interview with:
Leana Pande
Touro College of Osteopathic Medicine
MedicalResearch.com: What is the background for this study?
Response: Buprenorphine is not a new drug. It was developed in the 1960s with the intent of providing the benefits of opioids, without the addictive side effects. Unlike many prescription opioids,1 use of this Schedule III drug is increasing.2 It is often characterized as a partial agonist at the mu-opioid receptor (Figure-Right). Buprenorphine is available in many routes of administration and also with (brand name Suboxone) or without naloxone. Buprenorphine is a first-line pharmacotherapy for pregnant women with OUD.3 This review was completed in order for the benefits, and risks, of buprenorphine to be more fully appreciated and inform utilization for both opioid use disorder (OUD) and the treatment of pain.
MedicalResearch.com: What should readers take away from your report
Response: There were four key findings.4
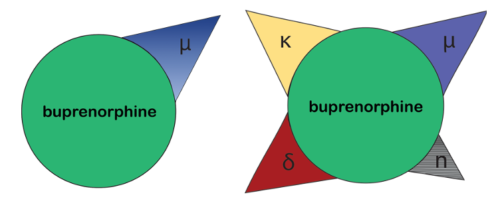 First, the pharmacodynamics and pharmacokinetics of this atypical opioid are much more complex than is commonly appreciated. Unlike the over-simplified version (right), it would be more accurate to describe the mechanism of action as a non-selective opioid modulator due to buprenorphine’s affinity for not only the mu, but also the delta and kappa, opioid receptors (left).
First, the pharmacodynamics and pharmacokinetics of this atypical opioid are much more complex than is commonly appreciated. Unlike the over-simplified version (right), it would be more accurate to describe the mechanism of action as a non-selective opioid modulator due to buprenorphine’s affinity for not only the mu, but also the delta and kappa, opioid receptors (left).
Second, norbuprenorphine is a potent metabolite.
Third, crowding sourcing from StreetRx.com has identified a buprenorphine street value (USD 3.95/mg), indicating appreciable non-medical use. There have also been eleven-thousand reports involving buprenorphine and children to US poison control centers.
Fourth, and perhaps most unanticipated, was the preclinical literature about the neurobehavioral risks of low and clinically relevant doses of buprenorphine when administered to pregnant rodents. This includes reductions in myelin and increases in depression-like behaviors.
There should be continued efforts to better understand the pharmacodynamics and pharmacokinetics of buprenorphine in order to better understand the uses for this agent. There is limited research on the full effect of the drug’s metabolites and method of administration, which may lead to incorrect perceptions on efficacy, safety, and diversion of this opioid. Buprenorphine has unmet potential for use in OUD and analgesia, including neuropathic pain, postoperative pain, persistent chronic pain, and cancer pain.
MedicalResearch.com: What recommendations do you have for future research as a results of this study?
Response: The neurobehavioral teratology of buprenorphine is under-appreciated by the general public and practicing clinicians.3 This should be both further investigated including with longitudinal structural and functional neuroimaging, but also more clearly communicated.3 There should be additional research about the most effective strategies to encourage patients to regularly keep controlled substances including buprenorphine in a locked storage box to prevent diversion. Buprenorphine has a few major metabolites and there is a limited amount of information available on their effects and receptor activity. It is assumed that a drug that is a partial agonist has limited activity, but further research in methods of administration and consideration for metabolite and concurrent receptor binding may provide more information regarding effects of buprenorphine. This has implications for clinical use of the drug and medications that can be co-administered with buprenorphine.
MedicalResearch.com: Is there anything else you would like to add? Any disclosures?
Response: As the number of opioid overdoses each year continues to increase, there is interest in whether a buprenorphine metabolite could be developed as an alternative to buprenorphine and potentially more effective OUD pharmacotherapy. It would be important to determine if any of the metabolites has a particular impact on analgesia and if this effect can be strengthened with different types of pain by using specific methods of administration (transdermal patches, buccal or oral formulations, etc.).
The last author was (2019-21) part of an osteoarthritis research team supported by Pfizer and Eli Lilly. The first two authors have no disclosures.
Citations:
- Piper et al. Trends in medical use of opioids in the U.S., 2006–2016. American Journal of Preventive Medicine 2018; 54(5):652-660.
- Hsu et al. An analysis of patterns of distribution of buprenorphine in the United States using ARCOS, Medicaid, and Medicare databases. Pharmacology Research Perspectives 2023;11(4):e01115.
- Seligman et al. Opioid use disorder: Overview of treatment during pregnancy. UpToDate. Accessed 9/30/2023.
- Pande et al. An examination of the complex pharmacological properties of the non-selective opioid modulator buprenorphine. Pharmaceutics 2023; https://www.mdpi.com/1424-8247/16/10/1397
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition.
Some links may be sponsored. Products are not endorsed.
Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
