Author Interviews, Dermatology / 09.07.2020
Dermatologist Discusses Patient Struggles with Facial Rosacea and Psoriasis
MedicalResearch.com Interview with:
Julie C. Harper, MD
Clinical Associate Professor of Dermatology
University of Alabama-Birmingham
MedicalResearch.com: What is the background for this study?
How common is rosacea?
What are the clinical manifestations of facial rosacea or psoriasis?
-
- In 2018, the initial Beyond the Visible report provided new insights into rosacea and the psychosocial burden associated with invisible symptoms. These insights further highlighted how little is really known of the burden faced by those suffering from facial skin diseases. In this new 2020 report, Beyond the Visible: rosacea and psoriasis of the face, we explored new dimensions of the burden faced by both rosacea and psoriasis of the face patients to learn from patients’ experiences and behaviors to better inform treatment based on the comparisons and contrasts. The survey spanned six countries, 300 rosacea patients and 318 psoriasis of the face patients to answer three questions:
- What is the burden faced by patients with psoriasis of the face and of rosacea?
- How does the burden faced by rosacea patients differ from patients with psoriasis of the face?
- What can we learn to help both patients and doctors to achieve the best possible outcomes for their patients?
- Rosacea is more common than people might initially assume. There are an estimated 16 million Americans with rosacea, building up to 415 million rosacea sufferers worldwide.
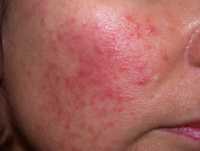
- Rosacea is commonly characterized by persistent redness and facial flushing, inflammatory lesions which may resemble acne-like bumps, visible blood vessels, and skin thickening. However, it’s important to note that rosacea symptoms vary person to person. Psoriasis of the face symptoms present themselves as red, scaly lesions that are usually along the forehead, hairline and ears. Moreover, psoriasis is known to be associated with itching, while rosacea sufferers have reported burning and stinging.
- In 2018, the initial Beyond the Visible report provided new insights into rosacea and the psychosocial burden associated with invisible symptoms. These insights further highlighted how little is really known of the burden faced by those suffering from facial skin diseases. In this new 2020 report, Beyond the Visible: rosacea and psoriasis of the face, we explored new dimensions of the burden faced by both rosacea and psoriasis of the face patients to learn from patients’ experiences and behaviors to better inform treatment based on the comparisons and contrasts. The survey spanned six countries, 300 rosacea patients and 318 psoriasis of the face patients to answer three questions: