How to Maintain Your Appearance After Plastic Surgery with Med Spa Treatments
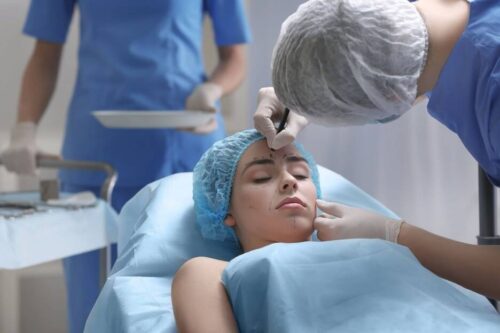

Since hair grows in cycles, multiple treatments are necessary to target all follicles effectively. For optimal results, most clients require...
When it comes to offering top-tier cosmetic treatments, finding dermal fillers wholesale at affordable prices is essential. Whether you’re a clinic owner, aesthetician, or medical professional, getting your supplies from reliable suppliers makes a huge difference. Let’s dive into the benefits, features, and important details you should know about dermal fillers before making a purchase.
 The acne problem affects millions of people in today's lifestyle. It is caused due to numerous reasons including hormone changes, poor lifestyle habits, and genetic problems. Effective management of this disorder often depends on finding the best dermatologist. The expertise and approach of a dermatologist greatly impact the treatment plan and outcomes for acne patients. From topical treatments to advanced therapies, a skilled dermatologist provides several treatment choices to help you have better skin and increase your confidence.
Certifications
Evaluating the credentials and experience is the initial aspect of choosing a dermatologist. A certified dermatologist undergoes significant training and must have expertise in identifying and treating skin diseases effectively. Evaluate their educational background, training, and any other qualifications pertinent to dermatology.
Investigating the dermatologist's standing in the medical community can also help you understand their professional position and the quality of treatment they deliver.
(more…)
The acne problem affects millions of people in today's lifestyle. It is caused due to numerous reasons including hormone changes, poor lifestyle habits, and genetic problems. Effective management of this disorder often depends on finding the best dermatologist. The expertise and approach of a dermatologist greatly impact the treatment plan and outcomes for acne patients. From topical treatments to advanced therapies, a skilled dermatologist provides several treatment choices to help you have better skin and increase your confidence.
Certifications
Evaluating the credentials and experience is the initial aspect of choosing a dermatologist. A certified dermatologist undergoes significant training and must have expertise in identifying and treating skin diseases effectively. Evaluate their educational background, training, and any other qualifications pertinent to dermatology.
Investigating the dermatologist's standing in the medical community can also help you understand their professional position and the quality of treatment they deliver.
(more…) Imagine stepping out of the shower and instantly feeling like your skin is made of pure silk—never having to shave or wax again.
That’s what laser hair removal may do for you. It’s the perfect choice for modern women with packed schedules. And on top of hair-free and smooth skin, it also boosts your confidence and saves you money and time.
It’s time to ditch those painful wax strips. Keep reading to find out why laser hair removal is the lifestyle upgrade you need.
Imagine stepping out of the shower and instantly feeling like your skin is made of pure silk—never having to shave or wax again.
That’s what laser hair removal may do for you. It’s the perfect choice for modern women with packed schedules. And on top of hair-free and smooth skin, it also boosts your confidence and saves you money and time.
It’s time to ditch those painful wax strips. Keep reading to find out why laser hair removal is the lifestyle upgrade you need.
Modern cosmetic treatments offer non-invasive options that can provide faster results for those looking to address deeper wrinkles, sagging skin,...
, understanding the process is crucial—results don’t appear immediately, so patience is necessary to see the full effects over time....
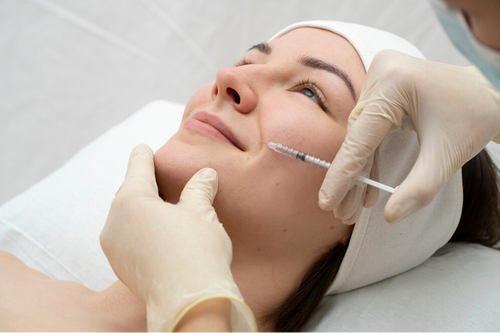 Many patients curious about fillers but unsure where to start. It's important to know that different fillers work best for different areas of the face. Some are great for plumping lips, while others excel at filling in deep lines around the mouth or eyes.
When thinking about getting fillers, it's crucial to talk with a skilled doctor. They can help you pick the right type for your goals and explain what to expect. Fillers can give quick results, but they don't last forever. Most need to be redone every few months to a year.
(more…)
Many patients curious about fillers but unsure where to start. It's important to know that different fillers work best for different areas of the face. Some are great for plumping lips, while others excel at filling in deep lines around the mouth or eyes.
When thinking about getting fillers, it's crucial to talk with a skilled doctor. They can help you pick the right type for your goals and explain what to expect. Fillers can give quick results, but they don't last forever. Most need to be redone every few months to a year.
(more…) Hannah Ceder
Department of Dermatology and Venereology
Institute of Clinical Sciences, Sahlgrenska Academy
University of Gothenburg
Gothenburg, Sweden
MedicalResearch.com: What is the background for this study?
Response: Many facial basal cell carcinomas (BCCs) are currently excised without prior biopsy, often resulting in incomplete surgical excisions. This practice is concerning because histopathologically high-risk BCCs have an increased risk of postoperative recurrence, necessitating a more meticulous surgical approach.
For facial high-risk BCCs, Mohs micrographic surgery is the recommended treatment method. Given these challenges, there is a clear need for simple, preoperative methods to help physicians identify high-risk tumors. By improving preoperative assessment, these methods could enhance treatment planning, reduce incomplete excisions, and optimize the use of Mohs micrographic surgery for high-risk cases.
(more…)
Hannah Ceder
Department of Dermatology and Venereology
Institute of Clinical Sciences, Sahlgrenska Academy
University of Gothenburg
Gothenburg, Sweden
MedicalResearch.com: What is the background for this study?
Response: Many facial basal cell carcinomas (BCCs) are currently excised without prior biopsy, often resulting in incomplete surgical excisions. This practice is concerning because histopathologically high-risk BCCs have an increased risk of postoperative recurrence, necessitating a more meticulous surgical approach.
For facial high-risk BCCs, Mohs micrographic surgery is the recommended treatment method. Given these challenges, there is a clear need for simple, preoperative methods to help physicians identify high-risk tumors. By improving preoperative assessment, these methods could enhance treatment planning, reduce incomplete excisions, and optimize the use of Mohs micrographic surgery for high-risk cases.
(more…)Alopecia or Hair Loss affects both men and women, although usually in different ways. The most common form of hair...
It is recommended to avoid direct sun exposure for the first several weeks after the procedure to protect his or...
 Eczema, also known as atopic dermatitis, is a chronic skin condition characterized by dry, itchy, and inflamed skin. While there are various treatments available, many people turn to home remedies to manage their symptoms. In this article, several natural remedies for eczema are explored, some remedies that should be avoided are discussed and tips for treating eczema in babies and children are identified.
Eczema, also known as atopic dermatitis, is a chronic skin condition characterized by dry, itchy, and inflamed skin. While there are various treatments available, many people turn to home remedies to manage their symptoms. In this article, several natural remedies for eczema are explored, some remedies that should be avoided are discussed and tips for treating eczema in babies and children are identified.
