Author Interviews, JAMA, Pharmaceutical Companies / 11.10.2024
McGill Study Finds Industry Payments Common to Peer Reviewers of Medical Journals
MedicalResearch.com Interview with:
David-Dan Nguyen MDCM MPH Doctoral Student Institute of Health Policy Management and Evaluation and Resident Physician Division of Urology University of Toronto
MedicalResearch.com: What is the background for this study? Response: Peer reviewers are crucial to the academic publishing process. While there’s been significant scrutiny of potential conflicts of interest among authors and editors of major journals, the potential for conflicts of interest among peer reviewers has been relatively unexplored. As such, our study aimed to quantify and characterize industry payments made to peer reviewers of top medical journals—The BMJ, JAMA, The Lancet, and The New England Journal of Medicine—to better understand the extent of these financial relationships. (more…)





 Lauren C. Davis, MBS
Department of Medical Education
Geisinger Commonwealth School of Medicine
Scranton, PA 19409
MedicalResearch.com: What is the background for this study?
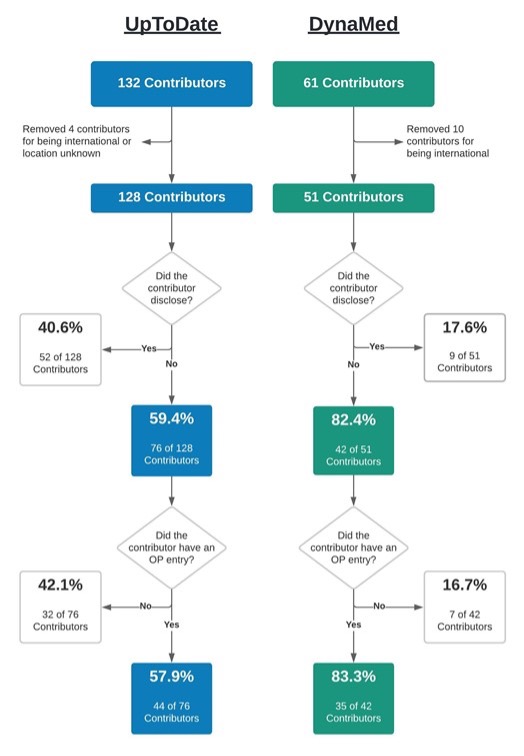
Response: Financial conflicts of interest (COIs) resulting from ties between academia and industry have been under scrutiny for their potential to hinder the integrity of medical research. COIs can lead to implicit bias, compromise the research process, and erode public trust (1-6). The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM), standardizes symptom criteria and codifies psychiatric disorders. This manual contributes to the approval of new drugs, extensions of patent exclusivity, and can influence payers and mental health professionals seeking third-party reimbursements. Given the implications of the DSM on public health, it is paramount that it is free of industry influence. Previous research has shown a high prevalence of industry ties among panel and task force members of the DSM-IV-TR and DSM-5, despite the implementation of a disclosure policy for the DSM-5 (7,8). This study (9) determined the extent and type of COIs received by panel and task-force members of the DSM-5-TR (2022) (10). As the DSM-5-TR did not disclose COI, we used the Center for Medicare and Medicaid Services Open Payments (OP) database (11) to quantify them.
Lauren C. Davis, MBS
Department of Medical Education
Geisinger Commonwealth School of Medicine
Scranton, PA 19409
MedicalResearch.com: What is the background for this study?
Response: Financial conflicts of interest (COIs) resulting from ties between academia and industry have been under scrutiny for their potential to hinder the integrity of medical research. COIs can lead to implicit bias, compromise the research process, and erode public trust (1-6). The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM), standardizes symptom criteria and codifies psychiatric disorders. This manual contributes to the approval of new drugs, extensions of patent exclusivity, and can influence payers and mental health professionals seeking third-party reimbursements. Given the implications of the DSM on public health, it is paramount that it is free of industry influence. Previous research has shown a high prevalence of industry ties among panel and task force members of the DSM-IV-TR and DSM-5, despite the implementation of a disclosure policy for the DSM-5 (7,8). This study (9) determined the extent and type of COIs received by panel and task-force members of the DSM-5-TR (2022) (10). As the DSM-5-TR did not disclose COI, we used the Center for Medicare and Medicaid Services Open Payments (OP) database (11) to quantify them.