
13 Apr A Novel Method to Examine the Varied Adverse Events of Commonly Prescribed Opioids in the United States
Medicalresearch.com Interview with:

Edward Liu
Edward Liu, BA
Second year medical student
Department of Medical Education
Geisinger Commonwealth School of Medicine
Scranton, PA
Medicalresearch.com: What is the background for this study?
Response: The United States’ opioid epidemic continues to rise because of increasing opioid use and availability, contributing to prescription opioid misuse, mortality, and rising cost.1 The worsening health and economic impact of opioid use disorder in the US warrants further attention on the adverse effects and distribution pattern of commonly prescribed opioids like oxycodone (OxyContin), fentanyl (Duragesic), and morphine (MS-Contin). Using the Automated Reports and Consolidated Ordering System (ARCOS) database,2 a comprehensive data collection system of pharmacies and hospitals distribution of Schedule II and III controlled substances in the US with the FDA Adverse Event Reporting System (FAERS)3 has never been done before. This approach may provide a more complete picture of the risks of prescription opioids which can include drowsiness, nausea, and potentially fatal respiratory depression.
Medicalresearch.com: What are the main findings?
Response: Almost seven-hundred thousand prescription opioid adverse drug events (ADE) submitted to FAERS were examined. The study4 found that oxycodone, fentanyl, and morphine accounted for over half of ADEs, while meperidine had a much lower incidence. Methadone consistently accounted for the largest proportion of opioid distribution but was consistently underrepresented for ADEs across all the three time periods (06’-10, ‘11-16, ‘17-21) when accounting for its distribution. The study also found that several opioids, including meperidine, oxymorphone, tapentadol, and hydromorphone, were overrepresented for ADEs in the most recent period (Figure).
This novel report highlights the need for continued monitoring and addressing of the ADEs associated with opioid use in the US. Incorporating FAERS and ARCOS provides an important new approach to evaluating post-marketing safety surveillance of these drugs and may inform healthcare policies and providers to better regulate the use of these prescription opioids.
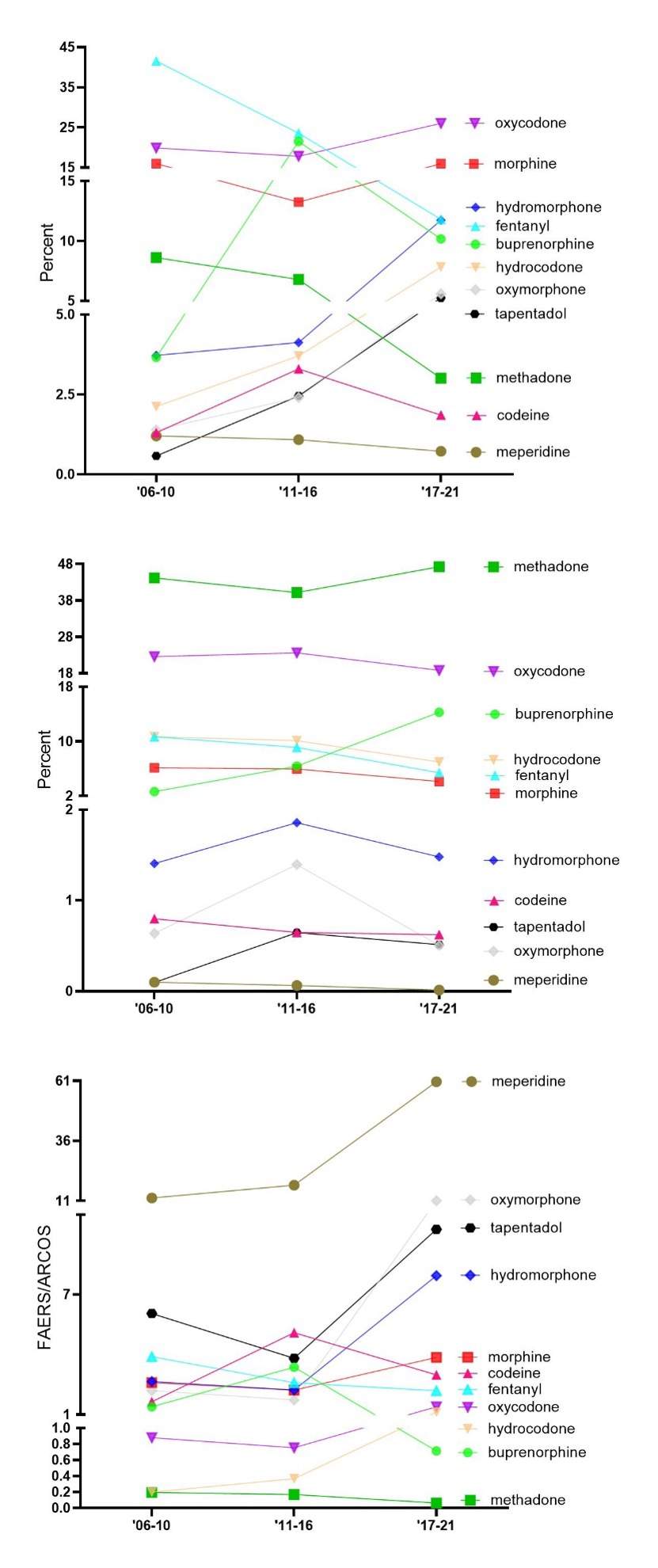
Figure. US Food and Drug Administration’s Adverse Event Report System (FAERS) prescription opioid adverse events from 2006 to 2021 (n = 667,969, top). Drug Enforcement Administration’s Automated Reports and Consolidated Ordering System (ARCOS) prescription opioid distribution in Morphine milligram equivalents (MME) (middle). Ratio of adverse event reports of select opioids as reported to FDA Adverse Event Reports (FAERs) relative to the percent distribution as obtained from Automated Reports and Consolidated Ordering System (ARCOS) (bottom). Ratios > 1 are overrepresented and < 1 are underrepresented.
Medicalresearch.com: What should readers take away from your report?
Response: The opioid epidemic in the United States continues to be a substantial public health issue, with ongoing high rates of opioid consumption, misuse, and mortality.1 Certain opioids, such as oxycodone, fentanyl, and morphine, were associated with a higher number of ADEs than others, such as meperidine. Methadone (Dolophine) consistently accounted for the largest proportion of opioid distribution but was underrepresented for ADEs across all three time periods analyzed when accounting for its wide-spread distribution. Conversely, meperidine (Demerol) was highly overrepresented for ADE when correcting for its modest distribution.5
There is substantial value of using the Food and Drug Administration’s Adverse Event Reporting System (FAERS) in combination with the Drug Enforcement Administration’s Automation of Reports and Consolidated Orders System (ARCOS) to better understand their associated ADEs relative to distribution. This new approach may inform healthcare policies, providers, and prescription opioid users, to better regulate the use of opioids and improve patient safety.
Medicalresearch.com: What recommendations do you have for future research as a result of this study?
Response: There is further need to monitor and address the ADEs of commonly prescribed opioids. Analyzing the distribution patterns of select drugs with FAERS and ARCOS at the state level might help better understand possible variations in distributions among the states, which may prompt further investigation into state-level policies to regulate opioid use. Analyzing such patterns at the state level might better bring attention to the use of certain opioids, inform opioid stewardship efforts to better minimize harms, and help other countries avoid the iatrogenic opioid epidemic ravaging the US.
Medicalresearch.com: Is there anything else you would like to add? Any disclosures?
Response: The FAERs2 and ARCOS3 databases are publicly available. These are important resources that could be utilized to improve public health by researchers and data journalists.
References:
- Lyden J, Binswanger IA. The United States opioid epidemic. Semin Perinatol. 2019;43(3):123-131. doi:10.1053/j.semperi.2019.01.001
- U.S. Food and Drug Administration (FDA). (2021). FDA Adverse Events Reporting System (FAERS) Public Dashboard. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard
- United States Drug Enforcement Administration (DEA). (2022). Division Control Division. ARCOS Retail Drug Summary Reports. https://www.deadiversion.usdoj.gov/arcos/retail_drug_summary/index.html
- Liu E, et al. Variations in adverse drug events of opioids in the United States. Frontiers of Pharmacology, https://www.frontiersin.org/articles/10.3389/fphar.2023.1163976/full
- Harrison L, et al. Pronounced declines in meperidine in the US: Is the end imminent? Pharmacy 2022. 10(6):154.
The information on Medicalresearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
