
23 Jan Skin Biopsies May Be Useful For Early Detection of Prion Infections
MedicalResearch.com Interview with:

Dr-Caughey
Byron Caughey, Ph.D.
Senior Investigator
Chief, TSE/prion Biochemistry Section
Laboratory of Persistent Viral Diseases
NIH/NIAID Rocky Mountain Laboratories .
Hamilton, MT 59840 USA
MedicalResearch.com: What is the background for this study? Would you briefly explain the significance of prion-induced diseases and why they have been difficult to diagnosis?
Response: Although prion diseases such as Creutzfeldt-Jakob disease (CJD) are rarer than many other neurodegenerative diseases in humans, they are rapidly fatal, untreatable and transmissible. Therefore it is important to be able to diagnose prion diseases as early as possible, not only to accurately inform patients, families and caregivers, but also to reduce risks of transmission and improve prospects for developing therapeutics.
Toward these goals, we have shown that our RT-QuIC prion seed amplification assays are highly accurate for diagnosing sporadic CJD using patients’ spinal fluid and/or nasal brushings in the clinical phase of disease. However, these particular specimens may not always be available, and it remains unclear how early they become RT-QuIC-positive in infected individuals in the months or years prior to the onset of overt clinical signs. We also showed recently that skin samples obtained post-mortem from sCJD patients are RT-QuIC positive.
In the current study, we determined how early prion seeds appear in the rodents infected with prions in order to gain clues as to whether analyses of skin biopsies might provide a means of early preclinical detection of prion infections in humans.
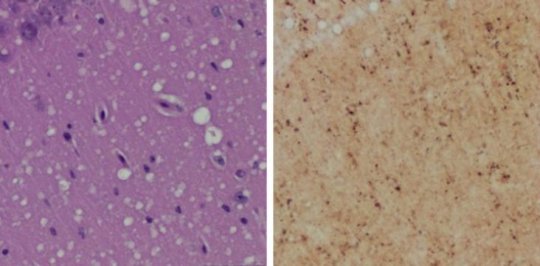
(Left) Staining shows spongiform degeneration. (Right) Staining shows intense misfolded prion protein.
Credit: Case Western Reserve University School of Medicine
MedicalResearch.com: What are the main findings?
Response: In many cases we detected prion seeding activity far in advance of clinical signs of disease in prion-infected rodents, as early as 2 weeks post-infection.
MedicalResearch.com: What should readers take away from your report?
Response: Our early detection of prion seeding activity in the skin of prion-infected rodents provides precedent for the possibility that prion seed amplification assays of skin biopsies could be helpful for early preclinical detection of prion infections in humans and other animals.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Efforts should be undertaken to determine how early and frequently prion seeding activity can be detected in humans, livestock and wildlife with prion diseases. Such studies should begin to assess the diagnostic utility of skin testing. Also future studies should address what parts of the skin contain prions, and, importantly, whether they ever appear on the skin’s surface.
Disclosure: I am named as an inventor on patent applications relating to RT-QuIC technologies.
Citation:
Zerui Wang, Matteo Manca, Aaron Foutz, Manuel V. Camacho, Gregory J. Raymond, Brent Race, Christina D. Orru, Jue Yuan, Pingping Shen, Baiya Li, Yue Lang, Johnny Dang, Alise Adornato, Katie Williams, Nicholas R. Maurer, Pierluigi Gambetti, Bin Xu, Witold Surewicz, Robert B. Petersen, Xiaoping Dong, Brian S. Appleby, Byron Caughey, Li Cui, Qingzhong Kong, Wen-Quan Zou. Early preclinical detection of prions in the skin of prion-infected animals. Nature Communications, 2019; 10 (1) DOI: 10.1038/s41467-018-08130-9
[wysija_form id=”3″]
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on January 23, 2019 by Marie Benz MD FAAD
