05 Jul TPOXXÒ (tecovirimat): Novel Antiviral Agent in the Event of a Smallpox Epidemic
MedicalResearch.com Interview with:
Dr. Dennis E. Hruby PhD
Chief Science Officer of SIGA Technologies
Corvallis, OR
MedicalResearch.com: What is the background for this study?
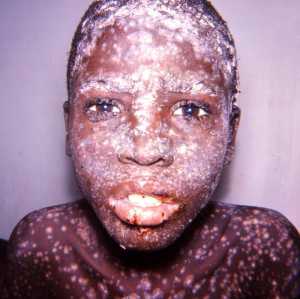
Response: Naturally occurring smallpox was declared eradicated in 1980 following coordinated decades-long global vaccination campaigns. However, there is a significant concern that smallpox, which is both highly contagious and highly lethal, could be used as a potential bioweapon.
DNA synthesis technology and the possibility of unaccounted for smallpox stocks pose significant risks. While there are two publicly acknowledged stocks of smallpox virus held by the United States and Russia, some believe that additional stores of the virus could be in the hands of governments or organizations that might use them to cause harm. The DNA sequence of the smallpox genome is in the public domain and could potentially be synthesized in a laboratory from scratch or created by genetically modifying a similar virus.
Currently, there are no therapies approved for the treatment of smallpox infection. A smallpox bioterror attack could be especially damaging because the majority of today’s population is not immune to the virus, as routine vaccination ended in the 1970s. It is estimated that without vaccination or treatment, each person infected with smallpox would infect 5 – 7 others. Rapid spread from person-to-person can occur through speaking, breathing or touching. Smallpox also can be transmitted by direct contact with infected fluids and contaminated objects. Furthermore, vaccination must occur within 3-5 days of exposure to smallpox, when patients are still asymptomatic, to be effective. These limitations underscore the need for an effective smallpox antiviral therapy, in addition to any available vaccine.
MedicalResearch.com: What are the main findings?
Response: TPOXXÒ (tecovirimat) is a novel, small molecule antiviral agent for the treatment of smallpox. With smallpox eradication in 1980, there are no naturally occurring smallpox outbreaks in which to test the efficacy of TPOXX in humans, and it would be unethical to intentionally infect trial participants with a virus that has shown mortality rates of as high as 30%. Consequently, TPOXX has been evaluated using a novel development path based on the FDA’s “Animal Rule,” in which safety studies are conducted in healthy human volunteers and efficacy and toxicology studies are conducted in animal models.
The key findings from the data published in New England Journal of Medicine are that the oral formulation of TPOXX demonstrated efficacy in two animal models of poxvirus infection (non-human primates and rabbits) and that no safety concerns were identified in association with the use of TPOXX in human volunteers who received a 14-day course of the drug. The data also confirm earlier observations from studies in nonhuman primates that efficacious drug exposures in the nonhuman primate model were higher than required in the rabbit model.
The minimum dose of TPOXX needed to achieve over 90% survival in non-human primates infected with a lethal dose of monkeypox virus was 10 mg/kg for 14 days, while a dose of 40 mg/kg for 14 days was similarly efficacious in rabbits infected with a lethal dose of rabbitpox virus. Based on these findings, the dose selected for safety testing in human volunteers was 600 mg twice daily for 14 days.
MedicalResearch.com: What should readers take away from your report?
Response: These data support the potential utility of TPOXX in the treatment of smallpox, and readers should know that the U.S. Government has added 2 million doses of oral TPOXX to the Strategic National Stockpile. In the event of a smallpox outbreak, the U.S. government would coordinate with public health officials in affected areas to determine the most effective response strategy and would make TPOXX available in conjunction with other medical countermeasures, as appropriate.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: These data support the safety and efficacy of oral TPOXX, and SIGA Technologies has submitted a New Drug Application to the U.S. Food and Drug Administration specifically for the use of TPOXX in the treatment of active smallpox disease. SIGA Technologies also has a development contract with the U.S. government for an intravenous formulation of TPOXX for use in individuals who cannot take TPOXX capsules.
Other future applications of TPOXX might include its prophylactic use in people not yet infected with smallpox but who are at risk of infection due to known exposure to an infected individual or proximity to the site of a smallpox outbreak. Additionally, data suggest that TPOXX also inhibits the replication and spread of other orthopoxviruses, such a monkeypox. Given that monkeypox infects humans and is associated with outbreaks in parts of Africa, there may be benefit in evaluating TPOXX in this indication as well. Of course, the use of TPOXX in these indications would require additional testing and appropriate regulatory approval.
MedicalResearch.com: Is there anything else you would like to add?
Response: SIGA Technologies has worked closely and extensively with the U.S. government during the development of TPOXX. This includes government funding of Research & Development as well as collaborating on experiments and discussions regarding development strategies. The most extensive work has been with the Biomedical Advanced Research and Development Authority (BARDA), but previous collaborations include the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the Defense Threat Reduction Agency (DTRA), and the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), in addition to close consultation with the FDA during the development of TPOXX. We believe that the development of TPOXX is an excellent example of how public-private partnerships can help address our country’s health security needs and catalyze innovation in the treatment of diseases with limited patient populations.
The studies reported in New England Journal of Medicine were funded by BARDA and by SIGA Technologies.
Citation:
Oral Tecovirimat for the Treatment of Smallpox
Douglas W. Grosenbach, Ph.D., Kady Honeychurch, Ph.D., Eric A. Rose, M.D., Jarasvech Chinsangaram, D.V.M., Ph.D., Annie Frimm, B.S., Biswajit Maiti, Ph.D., Candace Lovejoy, B.S., Ingrid Meara, M.S., Paul Long, B.S., and Dennis E. Hruby, Ph.D
July 5 2018
N Engl J Med 2018; 379:44-53
DOI: 10.1056/NEJMoa1705688
[wysija_form id=”3″]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on July 5, 2018 by Marie Benz MD FAAD
