09 Feb NEJM: New Oral Medication Addresses Common Itchy Back Condition
MedicalResearch.com Interview with:

Dr. Kim
Brian S Kim, MD, MTR
Sol and Clara Kest Professor
Vice Chair of Research | Site Chair, Mount Sinai West and Morningside
Director, Mark Lebwohl Center for Neuroinflammation and Sensation
The Kimberly and Eric J. Waldman Department of Dermatology
Precision Immunology Institute | Friedman Brain Institute
Icahn School of Medicine at Mount Sinai
MedicalResearch.com: What is the background for this study?
Response: We generated preliminary data in mice that difelikefalin suppresses itch robustly with very little to no effect on inflammation in a model of atopic dermatitis. This led us to hypothesize that the primary mode of action of difelikefalin must be by direct modulation of sensory neurons to suppress itch and thus would be best suited for a neuropathic itch condition. These are conditions in which itch occurs relatively independently of skin inflammation as in atopic dermatitis due to hyperactive nerves.
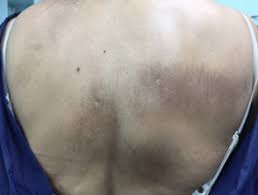
MedicalResearch.com: Would you briefly describe the condition of Notalgia Paresthetica?

Example of Notalgia paresthetica from Dermnet.nz
Response: Notalgia paresthetica is a common itch condition that presents in the middle of the back, likely due to minor nerve impingement. It is common and likely the reason the backscratcher was invented. The condition occurs more commonly likely due to our lifestyle in modern day which encourages poor posture on computers, desks, etc.
MedicalResearch.com: Is Difelikefalin used for any other conditions?
Response: Yes, for pruritus associated with chronic kidney disease in dialysis – this is an intravenous form of difelikefalin.
MedicalResearch.com: What are the main findings?
Response: Oral difelikefalin demonstrates significant clinically meaningful improvement compared to placebo in terms of itch improvement.
MedicalResearch.com: What should readers take away from your report?
Response: Notalgia paresthetica is common and should be suspected if you or someone you know suffers from an itchy back. There are no approved treatments for this kind of itch but this trial brings the hopeful possibility of a first of its kind treatment.
MedicalResearch.com: What recommendations do you have for future research as a results of this study?
Disclosures: I have consulted for Cara Therapeutics (manufacturer of difelikefalin) and our prelinical work was funded by Cara Therapeutics which led to this clinical trial.
Citation:
Phase 2 Trial of Difelikefalin in Notalgia Paresthetica
Brian S. Kim, M.D., M.T.R., Robert Bissonnette, M.D., Kristine Nograles, M.D., Catherine Munera, Ph.D., Nilam Shah, Pharm.D., Alia Jebara, M.D., Joshua Cirulli, Pharm.D., Joana Goncalves, M.D., and Mark Lebwohl, M.D. for the KOMFORT Trial Investigators
February 9, 2023
N Engl J Med 2023; 388:511-517 DOI: 10.1056/NEJMoa2210699
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on February 9, 2023 by Marie Benz MD FAAD
