Author Interviews, Cancer Research, NEJM, NIH, OBGYNE / 05.12.2024
NEJM: Prenatal cell-free DNA Can Detect Occult Maternal Cancer
MedicalResearch.com Interview with:
Diana W. Bianchi, M.D.
Senior Investigator
Center for Precision Health Research
Director,
Eunice Kennedy Shriver National Institute of Child Health and Human Development
National Institutes of Health
MedicalResearch.com: What is the background for this study?
Response: The ability of prenatal cell-free DNA (cfDNA) sequencing to incidentally detect maternal cancers has been demonstrated by several retrospective studies from commercial or national laboratories. However, there are no standardized approaches to the identification and medical management of prenatal screening results that might indicate a maternal cancer. We sought to prospectively identify DNA sequencing patterns and other biomarkers that could distinguish which women with nonreportable or unusual cfDNA sequencing results had cancer and to determine the best approach for diagnostic work-up of pregnant people who receive these results.
(more…)







 Prof. Hiddo Lambers Heerspink, PhD PHARMD
Department of Clinical Pharmacy and Pharmacology
University Medical Center Groningen
Groningen
Prof. Hiddo Lambers Heerspink, PhD PHARMD
Department of Clinical Pharmacy and Pharmacology
University Medical Center Groningen
Groningen












 Brett King, MD, PhD, FAAD
Associate Professor of Dermatology
Yale School of Medicine
MedicalResearch.com: What is the background for this study?
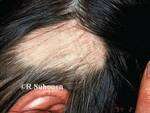
Response: Alopecia areata is an autoimmune disorder marked by disfiguring, non-scarring hair loss, and there are no therapies approved by the U.S. Food and Drug Administration for treatment of the disease. JAK inhibitors are showing promise for treatment of severe alopecia areata. In this work, the pooled results of two phase 3 clinical trials of the JAK inhibitor baricitinib were reported out to 52 weeks.
Brett King, MD, PhD, FAAD
Associate Professor of Dermatology
Yale School of Medicine
MedicalResearch.com: What is the background for this study?
Response: Alopecia areata is an autoimmune disorder marked by disfiguring, non-scarring hair loss, and there are no therapies approved by the U.S. Food and Drug Administration for treatment of the disease. JAK inhibitors are showing promise for treatment of severe alopecia areata. In this work, the pooled results of two phase 3 clinical trials of the JAK inhibitor baricitinib were reported out to 52 weeks.



