MedicalResearch.com Interview with:
Daniel Maloney, Ph.D
Farrar Lab
Smurfit Institute of Genetics
Trinity College Dublin
MedicalResearch.com: What is the background for this study? Would you briefly describe the condition of Dominant optic atrophy?
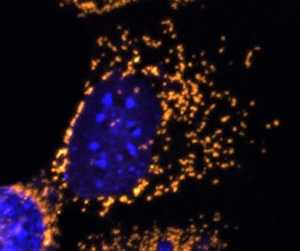
Response: Dominant Optic Atrophy (DOA) is a progressive blinding disorder that affects roughly 1:10,000 to 1:30,000 people. It is primarily caused by mutations in the OPA1 gene, which plays a pivotal role in the maintenance of the mitochondrial network. There is currently no way to prevent or cure DOA. We sought to build upon previous work to test if OPA1 could be delivered as a potential gene therapy intervention. (more…)
Author Interviews, Cancer Research / 03.10.2019
Wistar Researchers Identify Mitochondrial Factor That Keeps Cancer Cells Alive
 MedicalResearch.com Interview with:
Ekta Agarwal, Ph.D.
Postdoctoral fellow in the lab of
Dario Altieri, M.D.
Wistar president and CEO ,Director of the Institute’s Cancer Center
Robert & Penny Fox Distinguished Professor
and co-first author on the study.
MedicalResearch.com: What is the background for this study?
Response: Mitochondrial reprogramming is one of the hallmarks of cancer cell growth and metastasis. There are several studies correlating mitochondrial dynamics to increased cancer cell motility and invasion. However, therapies that can target molecular markers associated with mitochondrial functions and integrity are still obscure. Thus, it is crucial to identify novel targets and pathways that regulate mitochondrial functions in cancer. This study reveals one such mitochondrial molecular pathway which might serve as an actionable anti-cancer therapy.
(more…)
MedicalResearch.com Interview with:
Ekta Agarwal, Ph.D.
Postdoctoral fellow in the lab of
Dario Altieri, M.D.
Wistar president and CEO ,Director of the Institute’s Cancer Center
Robert & Penny Fox Distinguished Professor
and co-first author on the study.
MedicalResearch.com: What is the background for this study?
Response: Mitochondrial reprogramming is one of the hallmarks of cancer cell growth and metastasis. There are several studies correlating mitochondrial dynamics to increased cancer cell motility and invasion. However, therapies that can target molecular markers associated with mitochondrial functions and integrity are still obscure. Thus, it is crucial to identify novel targets and pathways that regulate mitochondrial functions in cancer. This study reveals one such mitochondrial molecular pathway which might serve as an actionable anti-cancer therapy.
(more…)
Aging, Author Interviews, Dermatology / 21.07.2018
Restoring Mitochondrial Function Reverses Wrinkles and Hair Loss – in Mice
MedicalResearch.com Interview with:
Keshav K. Singh, Ph.D.
Joy and Bill Harbert Endowed Chair in Cancer Genetics
Professor of Genetics, Pathology and Environmental Health
Founding Editor-in-Chief, Mitochondrion Journal
Director, Cancer Genetics Program
The University of Alabama at Birmingham
Birmingham, AL 35294
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Decline in mitochondrial DNA content and mitochondrial function has been observed in aging humans. We created mouse to mimic those condition to show that decline in mitochondrial function leads to development of wrinkles and loss of hair.
The main finding is that by restoring mitochondrial function we can reverse skin wrinkles to normal healthy skin and also regain hair growth. (more…)
 MedicalResearch.com Interview with:
Mary Kay Lobo, PhD
Associate Professor
University of Maryland School of Medicine
Department of Anatomy and Neurobiology
Baltimore, MD 21201
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Altered energy balance has been studied in drug abuse but the fundamental source of energy, mitochondria, has not been well examined. In this study we found that a molecular regulator of mitochondrial fission (division) is increased in the nucleus accumbens, a major brain reward region, of rodents exposed to repeated cocaine and postmortem samples of cocaine dependent individuals. We further found that mitochondrial fission is increased in a nucleus accumbens neuron subtype in rodents that self-administer cocaine. Pharmacological blockade of mitochondrial fission can prevent physiological responses to cocaine in this neuron subtype while reducing cocaine-mediated behaviors. Finally, genetic reduction of mitochondrial fission in this neuron subtype in the nucleus accumbens can reduce drug (cocaine) seeking in rodents previously exposed to cocaine. In contrast, increasing mitochondrial fission, in this neuron subtype, enhances cocaine seeking behavior.
(more…)
MedicalResearch.com Interview with:
Mary Kay Lobo, PhD
Associate Professor
University of Maryland School of Medicine
Department of Anatomy and Neurobiology
Baltimore, MD 21201
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Altered energy balance has been studied in drug abuse but the fundamental source of energy, mitochondria, has not been well examined. In this study we found that a molecular regulator of mitochondrial fission (division) is increased in the nucleus accumbens, a major brain reward region, of rodents exposed to repeated cocaine and postmortem samples of cocaine dependent individuals. We further found that mitochondrial fission is increased in a nucleus accumbens neuron subtype in rodents that self-administer cocaine. Pharmacological blockade of mitochondrial fission can prevent physiological responses to cocaine in this neuron subtype while reducing cocaine-mediated behaviors. Finally, genetic reduction of mitochondrial fission in this neuron subtype in the nucleus accumbens can reduce drug (cocaine) seeking in rodents previously exposed to cocaine. In contrast, increasing mitochondrial fission, in this neuron subtype, enhances cocaine seeking behavior.
(more…)
Author Interviews, Weight Research / 31.10.2017
Limiting Calories Changes Mitochondria, The Main Hub For Energy Metabolism
MedicalResearch.com Interview with:
Alicia J. Kowaltowski, MD, PhD
Professor of Biochemistry
Departamento de Bioquímica, IQ, Universidade de São Paulo
Cidade Universitária
São Paulo, SP, Brazil
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: We recently found that brain mitochondria from calorically-restricted animals can take up more calcium than mitochondria from animals that eat ad libitum (or "all they can eat"; doi: 10.1111/acel.12527). Calcium is a well-know regulator of energy metabolism, as is caloric intake, but this was the first evidence that limiting caloric intake changed calcium handling by mitochondria, the main hub for energy metabolism. As a result, we decided to investigate if this result was specific for the brain or happened in other tissues, focusing on the liver because of its central importance in metabolic control.
We found that liver mitochondria from calorically-restricted mice take up substantially more calcium than ad libitum fed mice. We also found that this result is related to a change in the amount of ATP within the mitochondria; ATP can complex calcium ions effectively due to its negative charges. Finally, we were able to correlate the increase in calcium uptake by liver mitochondria to a very strong protection of caloric restriction livers against ischemia/reperfusion damage.
(more…)
Author Interviews, Cancer Research, Nature, Wistar / 20.12.2016
A Neuronal Network of Mitochondrial Dynamics Regulates Cancer Metastasis
MedicalResearch.com Interview with:
Cecilia Caino, Ph.D.
The Wistar Institute
3601 Spruce Street
Philadelphia, PA 19104
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Mitochondria have recently experienced a resurgence of interest in the field of cellular biology. Traditionally known for their role in energy production and in programmed cell death, mitochondria are more broadly recognized as signaling hubs and biosynthetic factories. Not surprisingly, mitochondria have been linked to several hallmarks of cancer, including evasion of apoptosis, tissue invasion and metastasis and abnormal metabolic pathways. It has become clear that mitochondria quality control and metabolism-regulated shape changes are dysregulated in cancer. Recent studies identified a novel therapy-resistance mechanism that involves mitochondrial subcellular re-localization and is responsible for enhanced metastatic potential of cancer cells. In this context, the molecular regulators of mitochondrial trafficking in cancer are largely unknown.
Through analysis of shRNA screening results, we identified Syntaphilin (SNPH), which is considered to moderate mitochondrial trafficking in neurons, as a non-neuronal tissue specific factor to suppress cancer cell invasion. Using multi-disciplinary cell biological, real time imaging, in vivo studies and human clinical studies, SNPH was revealed to block cell motility and tumor metastasis by regulation of reprogramming of mitochondrial dynamics. We provided evidence from public databases and clinical samples that SNPH levels are decreased in different types of human tumors and low SNPH levels correlate with worse patient prognosis. Overall this study demonstrated a new mechanism by which tumor cell invasion is regulated by a SNPH-mediated pathway.
(more…)
Author Interviews, Memory, Scripps / 19.09.2016
Calcium Channels in Mitochondria Appears Critical To Capacity For Memory
MedicalResearch.com Interview with:
Ron L. Davis, PhD Professor and Chair
Department of Neuroscience
Florida campus of The Scripps Research Institute
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: While calcium’s importance for our bones and teeth is well known, its role in neurons—in particular, its effects on processes such as learning and memory—has been less well defined. Our new study, published in the journal Cell Reports, offers new insights how calcium in mitochondria—the powerhouse of all cells—can impact the development of the brain and adult cognition.
Specifically, we show in fruit flies, a widely used model system, that blocking a channel that brings calcium to the mitochondria called “mitochondrial calcium uniporter” causes memory impairment but does not alter learning capacity. That surprised us – we thought they wouldn’t be able to learn at all. This is important because defects in the same calcium channel function have been shown to be associated with intellectual disability in humans.
(more…)
Author Interviews, Fertility, OBGYNE, PNAS / 23.08.2016
Step Closer To Treating Mitochondrial Diseases by Understanding How Embryos Digest Sperm
MedicalResearch.com Interview with:
Peter Sutovsky PhD
Professor of Animal Science in the College of Agriculture, Food and Natural Resources
University of Missouri
Professor of Obstetrics, Gynecology and Women’s Health at the School of Medicine
University of Missouri Health System
MedicalResearch.com: What is the background for this study? What are the main findings?
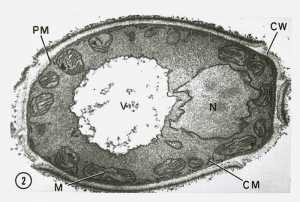
Response: Strictly maternal inheritance of mitochondria, the cellular power stations, and mitochondrial genes that mitochondria harbor, is a major biological paradigm in mammals. Propagation of paternal, sperm-contributed mitochondrial genes, resulting in a condition called heteroplasmy, is seldom observed in mammals, due to post-fertilization elimination sperm mitochondria, referred to as “sperm mitophagy.” Our and others’ recent results suggest that this process is mediated by the synergy of ubiquitin–proteasome system (UPS) pathway that recycles outlived cellular proteins one molecule at a time, and autophagic pathway capable of engulfing and digesting an entire mitochondrion.
Here we demonstrate that the co-inhibition of the ubiquitin-binding autophagy receptor proteins SQSTM1, GABARAP, and UPS, and the UPS protein VCP dependent pathways delayed the digestion of sperm mitochondria inside the fertilized pig egg. By manipulating said proteins, we created heteroplasmic pig embryos with both the paternal and maternal mitochondrial genes. Such animal embryos that could be used as a biomedical model to research and alleviate certain forms of mitochondrial disease.
(more…)