Author Interviews, Education, Medical Imaging, Technology / 13.08.2021
Temple First Year Med Students Provided with Butterfly iQ Point-of-Care Ultrasound Device
MedicalResearch.com Interview with:
Ryan C. Gibbons, MD, FAAEM, FACEP
Associate Professor of Emergency Medicine
Director of the Emergency Ultrasound Fellowship
Associate Director of the Division of Emergency Ultrasound
Department of Emergency Medicine
Director of Ultrasound in Medical Education
Lewis Katz School of Medicine at Temple University
MedicalResearch.com: What is the background for this study? How was the gift funded?
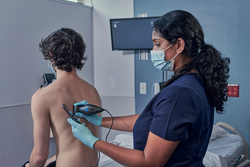
 Response: Point-of-care ultrasound is one of the most significant advances in bedside patient care, and its use is expanding across nearly all fields of medicine. In order to best prepare medical students for residency and beyond, it is imperative to begin POCUS training as early as possible. At the Lewis Katz School of Medicine at Temple University, we introduced POCUS education over a decade ago and have expanded it since then.
By providing each student with a Butterfly iQ device, we can augment our curriculum significantly. In addition to our robust pre-clinical sessions, now we will expand into the clinical years highlighting the utility of POCUS with actual patients.
This gift was made possible by the incredible generosity of Dr. Ronald Salvitti, MD ’63. (more…)
Response: Point-of-care ultrasound is one of the most significant advances in bedside patient care, and its use is expanding across nearly all fields of medicine. In order to best prepare medical students for residency and beyond, it is imperative to begin POCUS training as early as possible. At the Lewis Katz School of Medicine at Temple University, we introduced POCUS education over a decade ago and have expanded it since then.
By providing each student with a Butterfly iQ device, we can augment our curriculum significantly. In addition to our robust pre-clinical sessions, now we will expand into the clinical years highlighting the utility of POCUS with actual patients.
This gift was made possible by the incredible generosity of Dr. Ronald Salvitti, MD ’63. (more…)
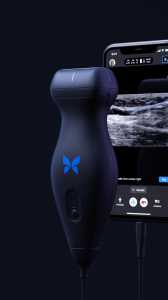 Response: Point-of-care ultrasound is one of the most significant advances in bedside patient care, and its use is expanding across nearly all fields of medicine. In order to best prepare medical students for residency and beyond, it is imperative to begin POCUS training as early as possible. At the Lewis Katz School of Medicine at Temple University, we introduced POCUS education over a decade ago and have expanded it since then.
By providing each student with a Butterfly iQ device, we can augment our curriculum significantly. In addition to our robust pre-clinical sessions, now we will expand into the clinical years highlighting the utility of POCUS with actual patients.
This gift was made possible by the incredible generosity of Dr. Ronald Salvitti, MD ’63. (more…)
Response: Point-of-care ultrasound is one of the most significant advances in bedside patient care, and its use is expanding across nearly all fields of medicine. In order to best prepare medical students for residency and beyond, it is imperative to begin POCUS training as early as possible. At the Lewis Katz School of Medicine at Temple University, we introduced POCUS education over a decade ago and have expanded it since then.
By providing each student with a Butterfly iQ device, we can augment our curriculum significantly. In addition to our robust pre-clinical sessions, now we will expand into the clinical years highlighting the utility of POCUS with actual patients.
This gift was made possible by the incredible generosity of Dr. Ronald Salvitti, MD ’63. (more…)