
07 Jul Pronounced state-level variation in Medicaid prescription of cannabinoids
MedicalResearch.com Interview with:

Edward Liu
Edward Liu, BA
Second year medical student
Department of Medical Education
Geisinger Commonwealth School of Medicine
Scranton, PA
MedicalResearch.com: What is the background for this study?
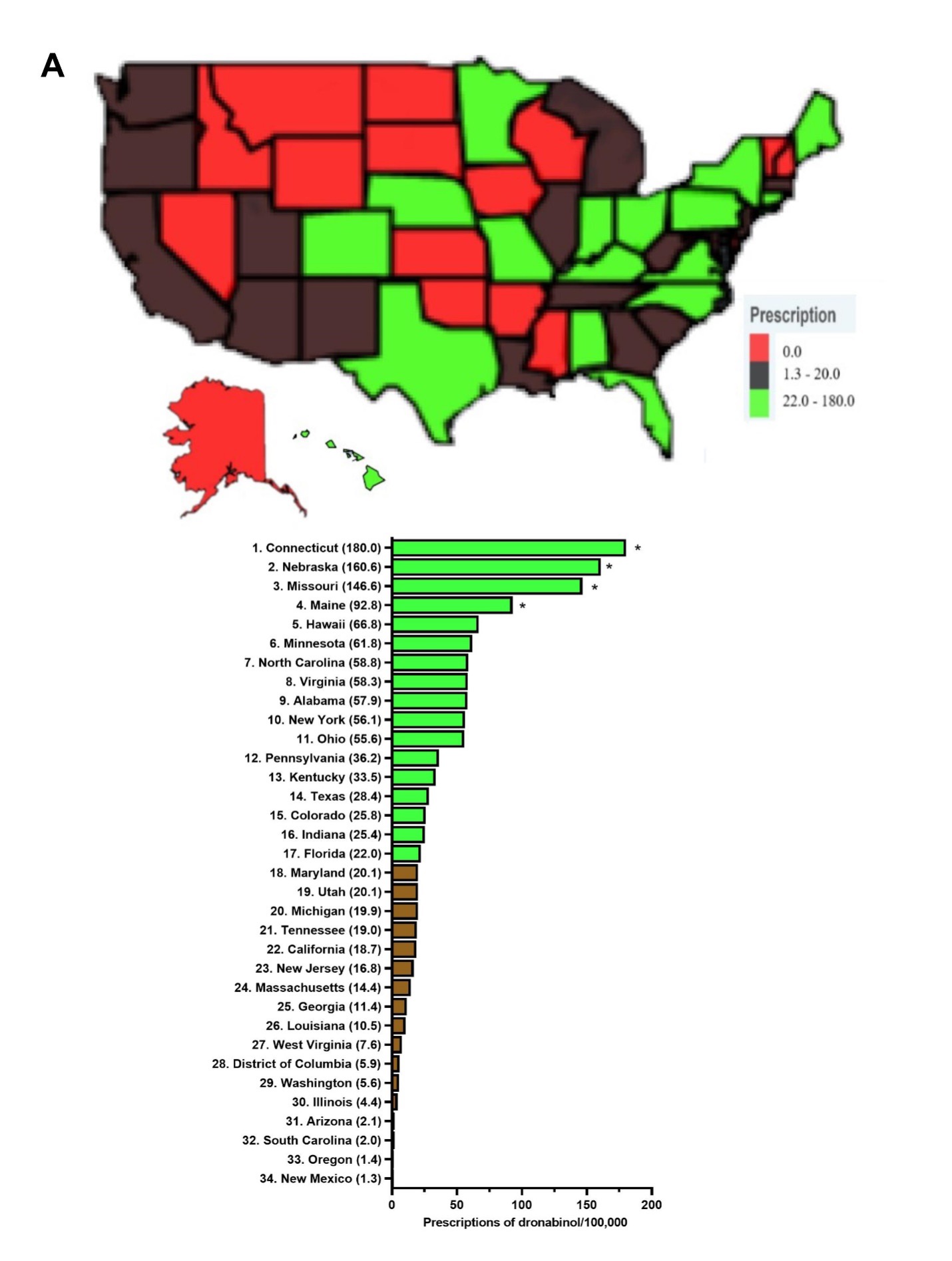
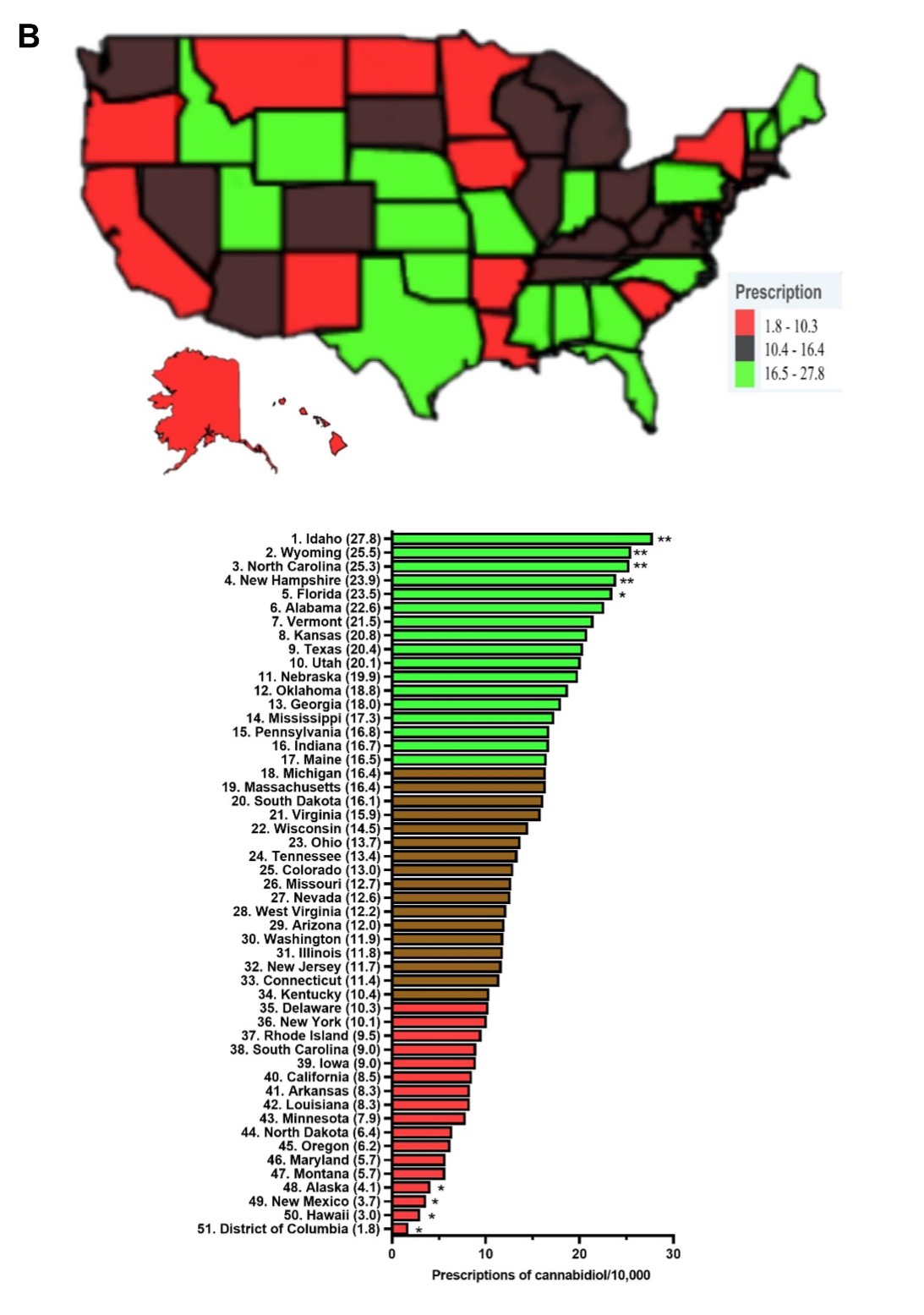
Response: The use pattern of two FDA approved cannabinoids, dronabinol (Marinol) and cannabidiol (Epidiolex) has not been previously studied. Dronabinol has been approved in the United States since 1985 for chemotherapy induced nausea as well as vomiting and HIV-induced anorexia,1,2 whereas cannabidiol has been approved since 2018 to treat childhood epileptic disorders, Lennox-Gastaut and Dravet syndrome.3 This longitudinal study examined Medicaid claims between 2016-2020 for these two prescription cannabinoids to better comprehend the state-level pharmacoepidemiologic trends and distribution of these drugs in US Medicaid amidst the increasing use of non-pharmaceutical formulations of cannabis.
MedicalResearch.com: What are the main findings?
Response: Dronabinol and cannabidiol demonstrated pronounced state-level disparities in prescriptions.4 Dronabinol average prescriptions per state decreased by a quarter (-25.3%) from 2016 to 2020, while that of cannabidiol showed a pronounced increase of 16,272.99 % from 2018 to 2020. When prescriptions are corrected for the number of enrollees, Connecticut, the state with the highest level of dronabinol prescriptions was 136.4 times larger than New Mexico. There was a total of seventeen states with zero prescriptions for dronabinol. As for cannabidiol, Idaho, the state with the highest prescriptions was significantly elevated relative to the national average and was 15.4-fold higher than the lowest, Washington DC. Furthermore, the Medicaid spending on these drugs parallels that of their prescription trend with a 66.3% decrease in reimbursement for dronabinol, while cannabidiol increased by +26,582.0%.
MedicalResearch.com: What should readers take away from your report?
Response: The prescriptions of dronabinol decreased while that of cannabidiol increased from 2016-2020, with both cannabinoids showing pronounced state level variations in prescribing to Medicaid patients. Many factors may contribute to the disparities, including state formularies, preferred drug list differences among states, prior authorizations, step therapies, and regulations may contribute to the drug reimbursements in Medicaid. Relative to the ubiquity of non-pharmaceutical grade marijuana products in the US including $4.6 billion for cannabidiol,4,5 Medicaid reimbursement for THC ($5.7 million) and cannabidiol ($233.3 million) was quite modest.
MedicalResearch.com: What recommendations do you have for future research as a results of this study?
Response: Further research may involve identifying the health policy or pharmacoeconomic origins of the state-level disparities of the two cannabinoids or including Medicare or privately insured US patients.
MedicalResearch.com: Is there anything else you would like to add? Any disclosures?
Response: The variation in distribution patterns of dronabinol and cannabidiol warrants further attention. Qualitative research may help better understand the decision-making process of health care providers and patients to use pharmaceutical or non-pharmaceutical grade cannabinoid products which may contribute to these pronounced disparities.
References
- Badowski ME, Yanful PK. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther Clin Risk Manag. 2018; 14:643-651.
- May MB, Glode AE. Dronabinol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics. Cancer Manag Res 2016; 8: 49-55.
- Abu-Sawwa R, Scutt B, Park Y. Emerging use of Epidiolex (cannabidiol) in epilepsy. J Pediatr Pharmacol Ther. 2020; 25(6): 485-499.
- Liu EY, Piper BJ, McCall KM. Pronounced state-level disparities in prescription of cannabinoids to Medicaid patients. Med Cannabis & Cannabinoids. 6(1): 58-65. https://pubmed.ncbi.nlm.nih.gov/37404688/
- Sill M. The future of the CBD industry in 2022 and beyond. 2021. Available from: https://www.forbes.com/sites/forbesbusinesscouncil/2021/10/21/the-future-of-the-cbd-industry-in-2022-and-beyond/?sh=3d3a016e25fd
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on July 7, 2023 by Marie Benz
