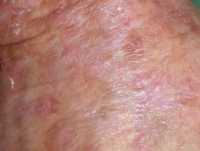
11 Feb Once Daily Five-Day Course of Novel Topical Agent Clears Actinic Keratoses
MedicalResearch.com Interview with:
Jane Fang, MD
Clinical
Athenex, Inc.
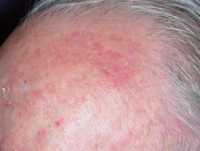
One example of actinic keratoses on scalp
DermNZ
MedicalResearch.com: What is the background for this study? Would you briefly explain what is meant by actinic keratoses? How common are they and who is primarily affected?
Response: Actinic keratosis is a very common precancerous skin condition that affects about 58 millions people in the US. Most commonly affected people are older (over 40 years old) men with fair skin type. Actinic keratosis lesions are red scaly bumps on sun-damaged skin mostly on the face, scalp, back of hands, forearms and legs. As there is a risk of 0.025-16% per year for each actinic keratosis to progress to skin cancer and it is not possible to predict which actinic keratosis will become cancerous, early treatment of actinic keratosis is generally recommended. Currently approved topical treatments require weeks or months of application and may lead to intolerable side effects that undermine compliance and reduce efficacy of treatment.
Tirbanibulin ointment is a novel anti-proliferative agent that inhibits tubulin polymerization and disrupts Src kinase signaling, and has the potential to inhibit growth of abnormal skin cells in actinic keratosis. The current report described two Phase 3 randomized vehicle or placebo-controlled clinical studies that demonstrated that a 5-day course of tirbanibulin ointment applied once daily by patients was safe, well-tolerated, and effective in clearing actinic keratosis on the face or scalp compared to vehicle control.
MedicalResearch.com: What are the main findings?
Response: The Phase III trials evaluated the efficacy and safety of tirbanibulin ointment 1% (10 mg/g) in adults with actinic keratosis on the face or scalp and included 702 patients across 62 sites in the United States. Patients were randomly assigned in a 1:1 ratio to receive tirbanibulin ointment or vehicle ointment, which was self-administered once daily for 5 consecutive days to a contiguous are of 25cm2 on the face or scalp containing 4-8 typical AK lesions.
Key findings are that the two Phase 3 trials met both the primary and secondary efficacy endpoints at day 57, and shown to be safe and well-tolerated.
For primary efficacy endpoint, a significantly higher percentage of patients on tirbanibulin had complete (100%) clearance of actinic keratosis than those on vehicle. Complete AK clearance rates with tirbanibulin vs. vehicle were 44% vs. 5% in trial 1 and 54% vs. 13% in trial 2, p<0.0001 in each trial.
Likewise, for secondary efficacy endpoint, a significantly higher percentage of patients on tirbanibulin cleared at least 75% of actinic keratosis in the treatment area by day 57, compared to those on vehicle. Partial clearance (≥75% reduction in lesions) rates with tirbanibulin vs. vehicle were 68% vs. 16% in trial 1 and 76% vs. 20% in trial 2, p<0.0001 in each trial
It was also found to be safe and well tolerated. Treatment compliance was over 99% for both trials. There was no discontinuation due to side effects or serious side effects.
Main side effects were mostly mild to moderate erythema and flaking or scaling, mild pain and pruritus at the application site. These reactions were transient, generally peaked by end of first week of use (day 8) and resolved spontaneously (i.e. without intervention) in about 2 weeks.
MedicalResearch.com: What should readers take away from your report?
Response: A 5-day course of once-daily self-applied tirbanibulin ointment is effective in clearing actinic keratosis. It is safe. Main side effects are transient mild to moderate local skin reactions that will resolve without intervention.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Future research that is being considered include clinical trials to evaluate the efficacy and safety of application over larger area, repeated courses of tirbanibulin ointment, and combination treatment with other approved forms of treatment for actinic keratosis.
Disclosure: tirbanibulin clinical program lead at Athenex (sponsor of Phase 3 clinical trials and developer of tirbanibulin).
Citation:
Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis
Andrew Blauvelt, M.D.,Steven Kempers, M.D., Edward Lain, M.D., Todd Schlesinger, M.D., Stephen Tyring, M.D., Ph.D., Seth Forman, M.D., Glynis Ablon, M.D., George Martin, M.D., Hui Wang, Ph.D., David L. Cutler, M.D., Jane Fang, M.B., B.S., and Min-Fun R. Kwan, M.B., B.S. for the Phase 3 Tirbanibulin for Actinic Keratosis Group*
February 11, 2021
N Engl J Med 2021; 384:512-520
DOI: 10.1056/NEJMoa2024040
[subscribe]
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on February 11, 2021 by Marie Benz MD FAAD
