08 Apr JAK Inhibitors Show Promise For Treatment of Severe Alopecia Areata
MedicalResearch.com Interview with:
 Brett King, MD, PhD, FAAD
Brett King, MD, PhD, FAAD
Associate Professor of Dermatology
Yale School of Medicine
MedicalResearch.com: What is the background for this study?
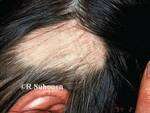
Response: Alopecia areata is an autoimmune disorder marked by disfiguring, non-scarring hair loss, and there are no therapies approved by the U.S. Food and Drug Administration for treatment of the disease. JAK inhibitors are showing promise for treatment of severe alopecia areata. In this work, the pooled results of two phase 3 clinical trials of the JAK inhibitor baricitinib were reported out to 52 weeks.
MedicalResearch.com: What are the main findings?
Response: In adults with severe alopecia areata (≥50% scalp hair loss) – average scalp hair loss was approximately 80%, and about half of patients had no scalp hair – treatment with baricitinib demonstrated scalp, eyebrow, and eyelash hair regrowth. Almost 40% and 25% of patients treated with baricitinib 4 mg and 2 mg, respectively, achieved ≤20% scalp hair loss during 52 weeks of treatment.
MedicalResearch.com: What should readers take away from your report?
Response: The JAK inhibitor baricitinib is effective for treatment of alopecia areata. Hopefully, we will soon have treatment to reverse this often awful disease!
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: We want to see results of longer treatment with baricitinib to see if efficacy continues to rise past 1 year.
MedicalResearch.com: Is there anything else you would like to add?
Response: Dr. Brett King has served on advisory boards and/or is a consultant and/or is a clinical trial investigator for Abbvie, AltruBio Inc, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz Therapeutics, Bristol-Meyers Squibb, Concert Pharmaceuticals Inc, Horizon Therapeutics, Eli Lilly and Company, Incyte Corp, LEO Pharma, Otsuka/Visterra Inc, Pfizer Inc, Regeneron, Sanofi Genzyme, TWi Biotechnology Inc, and Viela Bio. He is on speaker bureaus for Abbvie, Incyte, Leo Pharma, Pfizer Inc, Regeneron and Sanofi Genzyme.
Citation: AAD 2022 Abstract March 2022
- Long-Term Efficacy of Baricitinib in Patients with Severe Alopecia Areata: Week-52 Results from BRAVE-AA1 and BRAVE-AA2 Brett Andrew King, MD, PhD, FAAD
-
Two Phase 3 Trials of Baricitinib for Alopecia Areata
Brett King, M.D., Ph.D., Manabu Ohyama, M.D., Ph.D., Ohsang Kwon, M.D., Ph.D., Abraham Zlotogorski, M.D., Justin Ko, M.D., Natasha A. Mesinkovska, M.D., Ph.D., Maria Hordinsky, M.D., Yves Dutronc, M.D., Wen-Shuo Wu, M.D., Jill McCollam, Pharm.D., Chiara Chiasserini, Sc.D., Guanglei Yu, Ph.D., for the BRAVE-AA Investigators
NEJM: March 26, 2022
DOI: 10.1056/NEJMoa2110343
[wysija_form id=”3″]
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. Some links may be sponsored and no links are warranted or endorsed by MedicalResearch.com or its parent company, Eminent Domains Inc. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on April 8, 2022 by Marie Benz MD FAAD
