20 Jul KLISYRI® (tirbanibulin): Shorter Topical Treatment for Common Precancerous Actinic Keratoses
MedicalResearch.com Interview with:
Jane Fang, MD
Clinical
Athenex, Inc.
MedicalResearch.com: What is the background for this study?
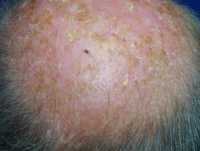
Response: Tirbanibulin is a first-in-class synthetic molecule that has potent anti-proliferative activity by inhibiting tubulin polymerization and disrupting src kinase signaling. It has been formulated as an ointment for the treatment of actinic keratosis, a very common precancerous condition of UV-damaged skin that affects over 50 million people in the US.
The most commonly adopted management approach is to remove AK lesions as it is hard to predict which lesion will become cancerous. Lesion-directed treatment like cryotherapy can effectively remove lesions one at a time but does not treat larger field of cancerization. Also, it is limited by associated pain and long term complication such as scarring. Currently approved topical treatments involve cumbersome application courses of weeks or months, and induce considerable local skin reactions that were not well tolerated by patients. The Phase 3 studies demonstrated that a short 5-day once daily course of tirbanibulin ointment 1% is an efficacious and safe topical treatment of actinic keratosis.
MedicalResearch.com: What are the main findings? How does this five-day treatment differ from other field therapies for AKs?
Response: The two Phase 3 randomized, placebo-controlled studies showed that tirbanibulin ointment 1% given once daily for 5 days was superior to placebo in clearing all AK lesions in the treated area of 25 cm2 in 49% (174/353 patients) of patients compared to 9% of patients (30/349 patients) after following for 8 weeks (p<.0.0001). Complete clearance was maintained in 27% of patients treated with tirbanibulin at 1-year follow-up.
All patients completed the 5-day treatment course except for one patient who missed the last dose and one patient who accidentally injured the treatment area. Adverse reactions were mostly mild application site itchiness and pain, mild to moderate erythema, flaking and scaling at the treatment area that resolved spontaneously. There were no serious adverse reactions nor early discontinuation or dose interruption related to tirbanibulin ointment use.
Even though no head-to-head comparison study has been conducted thus far, tirbanibulin ointment differentiates itself from other approved topical field therapies in 3 ways.
- Firstly, tirbanibulin ointment is applied over a short duration of 5 days compared to all other approved topical treatments that require weeks or months of treatment.
- Secondly, the complete clearance rates of tirbanibulin ointment appears consistent with other approved topical treatment for actinic keratosis in similar randomized placebo-controlled studies.
- Thirdly, tirbanibulin ointment is safe and well tolerated by patients, with mostly mild to moderate erythema, flaking and scaling that peak at around a week after starting treatment and resolve spontaneously.
MedicalResearch.com: What should readers take away from your report?
Response: Tirbanibulin ointment 1% when applied topically once daily for 5 consecutive days is an effective, safe, well tolerated treatment of actinic keratosis.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Actinic keratosis (AK) is a chronic skin condition. Recurrence and new occurrence of AK lesions in UV-damaged skin are inevitable even with effective and safe treatments. Future research should focus on treatment of larger field of skin involvement, sequential therapy (eg. retreatment with tirbanibulin ointment or sequential use of tirbanibulin ointment with other treatment modalities for AK), and long-term follow-up for durability of efficacy and safety.
MedicalResearch.com: Is there anything else you would like to add?
Response: With the approval of FDA in December 2020, tirbanibulin ointment 1% (KLISYRI), an effective, safe and convenient short course topical treatment of actinic keratosis, is now available as a prescription medication for patients.
Prescribing Information: LABEL (fda.gov)
Disclosures: All authors of “Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis” N Engl J Med 2021;384:512-20. DOI: 10.1056/NEJMoa2024040 are either participating investigators of the trials, or employees of Athenex Inc. Athenex, Inc. is the sponsor of the Phase 3 trials and with licensing partner Almirall S.A., are the manufacturers of tirbanibulin ointment 1%.
Citation:
11, 2021Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis
Blauvelt A., Kempers S., Lain E., et al.|N Engl J Med 2021; 384:512-520
JOIN OUR EMAIL LIST
[mailpoet_form id="5"]We respect your privacy and will never share your details.
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on July 20, 2021 by Marie Benz MD FAAD
