01 Jun Vitiligo: Off Label Topical Calcineurin Inhibitors Help Some Patients
MedicalResearch.com Interview with:
Jung Min Bae, MD, PhD
Associate Professor, Department of Dermatology
St. Vincent’s Hospital
College of Medicine, The Catholic University of Korea
MedicalResearch.com: What is the background for this study?
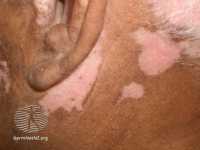
Response: Vitiligo is a common chronic skin disease affecting 1% of the population, and it causes low self-esteem and social stigma. To date, there are no approved drugs for the treatment of vitiligo, even though growing evidence indicates favorable therapeutic responses of topical calcineurin inhibitors (TCIs) including tacrolimus and pimecrolimus.
In this study, we conducted a systematic review and meta-analysis of all relevant prospective studies (n = 46) and identified remarkable therapeutic responses of TCI monotherapy and TCI plus phototherapy for vitiligo.
MedicalResearch.com: What are the main findings?
Response: Topical calcineurin inhibitors monotherapy produced an at least mild response in 55% of the patients and a marked response in 18% after a median treatment duration of 3 months. In a subgroup analysis, a marked response was achieved in 32% of children, and 35% of lesions on the face and neck. Also, combining TCI with phototherapy has been shown to considerably enhance the treatment response compared to TCI monotherapy or phototherapy alone.
MedicalResearch.com: What should readers take away from your report?
Response: Topical calcineurin inhibitors monotherapy has beneficial therapeutic effects, especially in children and in face and neck lesions. Therefore, Topical calcineurin inhibitor monotherapy is worth attempting to treat face and neck lesions, particularly in children, when phototherapy is not available. Moreover, as phototherapy and TCI treatment have the synergistic effects, TCI treatment should also be encouraged in vitiligo patients undergoing phototherapy. Given the long duration of vitiligo treatment and possible adverse effects of long-term topical corticosteroid use, Topical calcineurin inhibitor must be a critical element for vitiligo treatment.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Currently, Topical calcineurin inhibitor is only used off-label, because it is not approved for use in patients with vitiligo by regulatory agencies including the United States Food and Drug Administration (FDA), and the European Medicines Agency (EMA). Nonetheless, TCI has been used safely for nearly two decades, and its efficacy has been well proven in numerous studies. At this point, we reaffirmed the efficacy and safety of TCI for vitiligo through this systematic review.
It would be of great help to many patients with vitiligo if the regulatory authorities would consider approving the use of TCIs in vitiligo treatment. For vitiligo patients, more attention should be paid by physicians, pharmaceutical companies, and government authorities.
Citation:
Lee JH, Kwon HS, Jung HM, et al. Treatment Outcomes of Topical Calcineurin Inhibitor Therapy for Patients With Vitiligo: A Systematic Review and Meta-analysis. JAMA Dermatol. Published online May 29, 2019. doi:10.1001/jamadermatol.2019.0696
https://jamanetwork.com/journals/jamadermatology/article-abstract/2734015
[wysija_form id=”3″]
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on June 1, 2019 by Marie Benz MD FAAD

