19 Jun Most Patients with Cellulitis May Not Need IV Antibiotics
MedicalResearch.com Interview with:

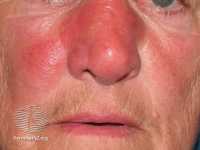
Example of cellulitis erysipelas from DermnetNZ.org
Richard Brindle DM FRCP
Honorary Reader, University of Bristol, UK
MedicalResearch.com: What is the background for this study?
Response: This review is an update of the 2010 Cochrane Review of Interventions for cellulitis and erysipelas (DOI: 10.1002/14651858.CD004299) but focusing on antibiotics. It provides a valuable resource for clinicians in summarizing current best evidence and highlighting gaps in the research. This review will inform the production of evidence-based guidelines covering antibiotic choice, route of administration, duration of treatment and the role of combinations of antibiotics.
MedicalResearch.com: What are the main findings?
Response: A total of 43 studies with a total of 5999 evaluable participants are included. Cellulitis was the primary diagnosis in only 15 studies as most studies were trials of antibiotics for skin and soft tissue infections. Overall, no evidence was found to support the superiority of any one antibiotic over another, and antibiotics with activity against methicillin-resistantStaphylococcus aureus did not add an advantage. Use of intravenous antibiotics over oral antibiotics and treatment duration of longer than five days were not supported by evidence. The use of combination therapy is not supported, as the trials with combination therapy do not demonstrate better outcomes.
MedicalResearch.com: What should readers take away from your report?
Response: The key message is that patients with cellulitis, and erysipelas, rarely need parenteral therapy and that an oral penicillin is as good as any other antibiotic. The patient’s outcome is not improved by courses of antibiotics longer than five days and combinations of antibiotics offer no benefit and simply increase the risk of adverse events.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Trials about cellulitis should include only participants with cellulitis and are needed to clarify the duration of antibiotic therapy, antibiotic dosing and whether longer durations are necessary with more severe disease.
Randomized controlled trials should be carried out comparing intravenous versus appropriate oral antibiotics for participants within a community setting, as this would have implications for delivering a more cost-effective home therapy that does not involve a home intravenous service or frequent outpatient hospital visits.
Trials need to have standardized criteria for severity scoring and a standardized set of outcomes needs to be established for these trials. These should include systemic features, local, blood and patient-focused parameters. Trial exclusions and times of follow up should be standardized wherever possible.
We have no conflicts of interest to declare.
Citation:
[wysija_form id=”3″]
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on June 19, 2019 by Marie Benz MD FAAD
