14 Dec NEJM: NYU Study Finds Significant Mpox Antibody Titers After Vaccination
MedicalResearch.com Interview with:

Dr. Angélica Cifuentes Kottkamp
Angélica Cifuentes Kottkamp, MD
Assistant Professor of Medicine
NYU Grossman School of Medicine
Associate Program Director
Infectious Diseases & Immunology Fellowship
Associate Director for Research & Diversity
NYU Langone Vaccine Center & VTEU
Attending Physician
H+H Bellevue Virology Clinic
Division of Infectious Diseases & Immunology
NYU Grossman School of Medicine
MedicalResearch.com: What is the background for this study? How does the JYNNEOS vaccine differ from the smallpox vaccine?
Response: JYNNEOS vaccine is a smallpox vaccine that was repurposed for Mpox given the similarities between the two viruses (smallpox and mpox). The vaccine (JYNNEOS) had been studied in people without HIV therefore there was a gap in knowledge in how this vaccine, especially the small dose (intradermal dose), would work in patients with HIV.
These patients resulted to be the most affected by the mpox outbreak suffering the worse outcomes of the disease with the highest death rates.
MedicalResearch.com: How many doses of the vaccine are optimal to confer immunity?

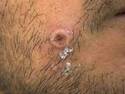
Example of MPox Dermnet Image
Response: The vaccine is currently a two-dose vaccine. Our study shows that two doses produce higher antibody responses, however, we found out that the median half-life (meaning, the average amount of time the antibodies are detectable in blood) was only 107 days. This suggests that further research is needed to clarify if future doses of the vaccine will be needed.
It is important to note, that this vaccine was designed to prevent smallpox, so maybe we just need a vaccine that is more specific to mpox in order to confer better and longer protection. Also, the detection of these antibodies is only one part of our complex immune system response! so more studies that look into a more comprehensive way to understand immune responses to vaccination are needed.
MedicalResearch.com: What are the adverse side effects? Can the vaccine be safely administered to patients with skin diseases ie eczema or psoriasis?
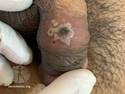
Example of MPox Dermnet Image
Response: The mpox vaccine is safe and well tolerated, some people can experience general reactions that are common to all vaccines such as pain at site of injection, fever, fatigue, etc.. however, these for the most part are transitory and making this vaccine very well tolerated. It is important to note that with the intradermal route of administration (the route that delivers the vaccine between the layers of the skin at a smaller dose) pain and skin discoloration or hyperpigmentation was noted. The vaccine is currently approved to be delivered via subcutaneous (under the skin) which is better tolerated than the intradermal route.
MedicalResearch.com: Is there anything else you would like to add?
Response: We are excited to share these findings with the medical community, public health officials, community organizations and with our patients. Mpox outbreaks are still occurring, just few weeks ago a new outbreak of Mpox was reported in the Democratic Republic of Congo. We need to be aware of what’s going on outside the U.S. and be prepared. Vaccines are excellent tools that we have, this is a great example of how good these vaccines are in different populations.
Citation:
Antibody Titers against Mpox Virus after Vaccination
December 14, 2023
N Engl J Med 2023; 389:2299-2301
DOI: 10.1056/NEJMc2306239
Angelica C. Kottkamp, M.D., Marie I. Samanovic, Ph.D., Ralf Duerr, M.D., Ph.D.
Aaron L. Oom, Ph.D., Hayley M. Belli, Ph.D. Jane R. Zucker, M.D.
Jennifer B. Rosen, M.D., Mark J. Mulligan, M.D.
https://www.nejm.org/doi/10.1056/NEJMc2306239
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition.
Some links may be sponsored. Products are not endorsed.
Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on December 14, 2023 by Marie Benz MD FAAD
