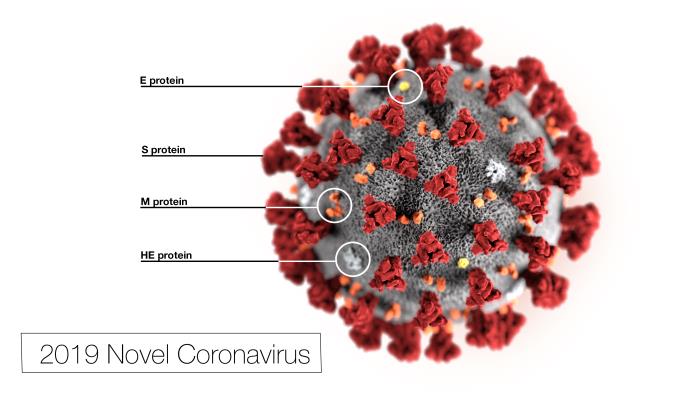
MedicalResearch.com Interview with:
Ariel Israel, M.D., Ph.D.
Director, Leumit Health Services
Tel Aviv, Israel
MedicalResearch.com: What is the background for this study?
Response: As a research institute of Leumit, one of the four state mandated health funds in Israel, we pursue research projects aimed at improving the health of our members, and reducing the burden of disease. For this purpose, we harness the unique resource of the electronic health records of our members, that is available in a central data warehouse for research purposes.
Israel was one of the first countries to roll-out a large-scale vaccination campaign, and to achieve control of the pandemics through vaccination. Nevertheless, since the middle of June '21, we have observed a gradual increase in the rate of COVID-19 infections among our members, even among the vaccinated. This increase was first believed to be due to the emergence of the delta strain, but when we compared vaccinated individuals who suffered from breakthrough infections to other vaccinated individuals, we found that the time that has elapsed since vaccination was significantly longer for individuals who got infected with COVID-19, in each of the age groups.
This prompted us to investigate the issue of a possible waning effect of the vaccine protection with time, that we present in this report, using the test negative study design.
We examined the electronic health records for 80,057 adults (average age 44 years) who received a PCR test at least three weeks after their second injection, and had no evidence of previous covid-19 infection. Of these 80,057 participants, 7,973 (9.6%) had a positive test result. These individuals were then matched to negative controls of the same age and ethnic group who were tested in the same week.
(more…)
 COVID-19 is a virus that devastated the healthcare systems around the globe. The main reason for this devastation was the speed of the spreading. Because it was spreading so fast, hospitals weren’t able to accommodate so many patients.
We needed to figure out a better approach to dealing with the pandemic. So, this is where most of the people on Earth stood together by being apart. We were in lockdown, but our scientists and governments collaborated more on finding the right solution.
COVID-19 is a virus that devastated the healthcare systems around the globe. The main reason for this devastation was the speed of the spreading. Because it was spreading so fast, hospitals weren’t able to accommodate so many patients.
We needed to figure out a better approach to dealing with the pandemic. So, this is where most of the people on Earth stood together by being apart. We were in lockdown, but our scientists and governments collaborated more on finding the right solution.