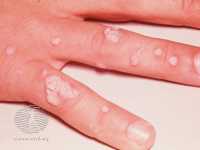
Author Interviews, COVID -19 Coronavirus, Herpes Viruses, HIV, HPV, Infections, STD / 24.04.2023
Thermo Fisher Scientific Works to Combat Risking STI Epidemic with Better, Faster Testing
MedicalResearch.com Interview with:
Manoj Gandhi, MD PhD
Senior Medical Director of Genetic Testing Services, Thermo Fisher Scientific.
Dr. Gandhi has been working to advance the quality of medical care globally. Using his knowledge of Clinical Medicine and Molecular Biology/Pathology, he is focused on bridging these two fields and bringing innovative solutions that help advance science, the practice of medicine with the ultimate goal of impacting patient lives, whether it be in Infectious Diseases or Oncology or Personalized Medicine. This approach allows him to explore creative ways to utilize technology to help better identify diseases and improve the direction and value of treatment.
MedicalResearch.com: What are the most common STIs prevalent in the US and worldwide today?
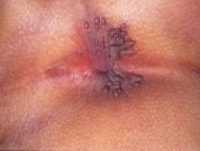
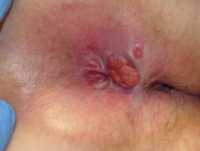
Response: By far, the most common STIs in the US and worldwide is Human Papillomavirus (HPV) that can cause cervical cancer in women and Herpes Simplex Virus (HSV) that is the cause of genital herpes. Outside of these two major causes of STI, the others that are very common are Chlamydia, Gonorrhea, Trichomoniasis and Syphilis. It is important to note that the reported cases represent only a subset of the individuals with an infection as many may be asymptomatic and could be spreading these STIs to others. HIV is another STI that is common but usually rests in its own category due to its impact.
(more…)






 Helen Trottier Ph.D
Assistant Professor, Department of Social and Preventive Medicine,
Researcher, CHU Sainte-Justine Research Center
Université de Montréal
Montréal, Québec, Canada
MedicalResearch.com: What is the background for this study?
Response: We know that HPV infection can have serious consequences such as the development of cancerous lesions in the cervix. HPV infection is also very prevalent in young women of childbearing age but the possible consequences of HPV in pregnancy have been poorly studied. Some population registers around the world have shown a reduction in the risk of preterm birth with HPV mass vaccination, but we must be careful with this kind of ecological correlation.
We have set up a large cohort study in pregnant women to study the association between HPV in pregnancy and preterm birth by targeting certain HPV genotypes and the duration of the infection.
Helen Trottier Ph.D
Assistant Professor, Department of Social and Preventive Medicine,
Researcher, CHU Sainte-Justine Research Center
Université de Montréal
Montréal, Québec, Canada
MedicalResearch.com: What is the background for this study?
Response: We know that HPV infection can have serious consequences such as the development of cancerous lesions in the cervix. HPV infection is also very prevalent in young women of childbearing age but the possible consequences of HPV in pregnancy have been poorly studied. Some population registers around the world have shown a reduction in the risk of preterm birth with HPV mass vaccination, but we must be careful with this kind of ecological correlation.
We have set up a large cohort study in pregnant women to study the association between HPV in pregnancy and preterm birth by targeting certain HPV genotypes and the duration of the infection.