
04 Jun ASCO: Antibody-Drug Conjugate Shows Promise in HER2 Positive Advanced Colorectal Cancer
MedicalResearch.com Interview with:

Dr. Siena
Salvatore Siena, MD
Director, Falck Division of Medical Oncology
Department of Hematology and Oncology, and Niguarda Cancer Center
Grande Ospedale Metropolitano Niguarda, Milano, I
Full Professor of Medical Oncology, Department of Oncology and Hemato-Oncology
Università degli Studi di Milano
MedicalResearch.com: What is the background for this study?
Response: There remains a significant unmet clinical need in treating patients with HER2 positive advanced colorectal cancer (CRC) who progressed on previous therapies. Exploratory clinical studies in CRC with HER2-amplification documented that patients with tumors with this molecular characteristic may benefit from HER2-targeted therapies (reviewed in Siena S et al Ann Oncol 2018). In particular, the phase 1 DS8201-A-J101 dose-expansion study of the cohort of patients with HER2 expressing non-breast/non-gastric or HER2 mutant solid tumors who received the 6.4 mg/kg dose of T-DXd, there were 20 patients with CRC. In this studythe experimental drug T-DXd (trastuzumab deruxtecan) showed clinical benefit and manageable safety profile.
- Investigator-assessed ORR (Objective Response Rate) of 15.0% (95% CI, 3.2-37.9), DCR (Disease Control Rate) of 80.0% (95% CI, 56.3-94.3), and median PFS (Progression Free Survival) of 4.1 months (95% CI, 2.1-5.9) was reported
- Common TEAEs (Treatment Emergent Adverse Events) include gastrointestinal (low grade) and hematological, which is consistent with overall T-DXd safety profile across various tumors
- ILD (Interstitial Lung Disease) was reported in 2 patients (Tsurutani et al. 2020)
Given the unmet need in the treatment of patients with HER2 positive advanced CRC who progressed on previous therapies and the clinical observations from the phase 1 DS8201-A-J101 study (see previous paragraph) , the phase 2 DESTINY-CRC01 trial was conducted to evaluate the efficacy and safety of T-DXd in patients with HER2 expressing CRC who were previously treated with at least 2 lines of therapy.
MedicalResearch.com: What are the main findings?
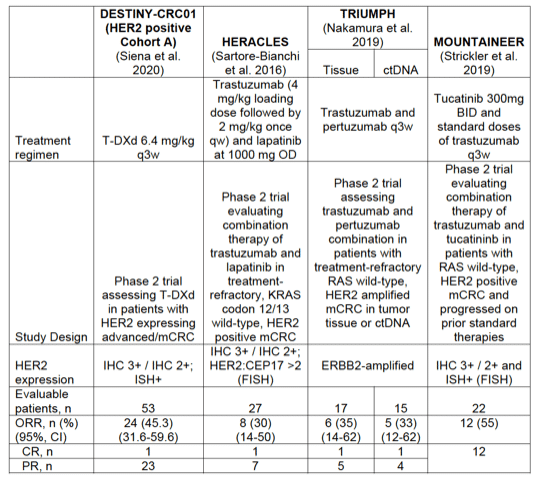
Response: The table below summarizes the patient population (HER2 criteria) and the responses observed in trials assessing HER2 directed therapies in CRC. There are currently no results available for head-to-head trials of T-DXd vs other HER2 directed therapies.
MedicalResearch.com: What should readers take away from your report?
Response: DESTINY-CRC01 study documents that T-DXd is a new powerful therapy against that 3% to 5% of advanced CRC that are addicted to HER2 oncogene that is amplified and thus leads to tumor progression (HER2-positive CRC). Interestingly, patients of the DESTINY-CRC01 study had progressed to standard therapies including irinotecan, oxaliplatin, fluoropyrimidimes, and anti-EGF monoclonal antibodies in 100% of cases and including a variety of anti-HER2 therapies in 30% of cases. Clinical benefit was implicit in the primary endpoint of the study that was confirmed objective response rate (ORR), also called a tumor response rate, which was assessed by independent central review, showed 45.3% of patients with HER2 positive advanced colorectal cancer (defined as IHC 3+ or IHC 2+/ISH+) treated with ENHERTU monotherapy (6.4mg/kg) achieved a tumor response. A disease control rate (DCR) of 83.0% was observed with a median progression-free survival (PFS) of 6.9 months. Median duration of response (DoR) and overall survival (OS) have not yet been reached at the time of data cut-off.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: T-DXd has now been shown to be a powerful therapy for HER2+ advanced CRC after failure of standard therapies and its role in the strategy of treatment of colorectal cancer should be further investigated in earlier lines of therapy. My own opinion is that T-DXd deserves evaluation in the second line therapy of metastatic CTC versus FOLFIRI or FOLFOX and either bevacizumab or panitumumab or cetuximab or aflibercept as per physicians choice. Another issue that in my opinion deserves further investigations also the evaluation of lower dose that that employed in the DESTINY CRC01 such as 5.4 mg/kg (“gastric or breast dose”) versus the present 6.4 “CRC dose”.
Disclosures: I have been a member of advisory boards for Amgen, Bayer, BMS, CheckmAb, Clovis, Daiichi-Sankyo, Merck, Roche-Genentech, and Seattle Genetics;
Citation: ASCO 2020 Virtual Meeting Oral Presentation
A phase II, multicenter, open-label study of trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): DESTINY-CRC01
JOIN OUR EMAIL LIST
[mailpoet_form id="5"]We respect your privacy and will never share your details.
Last Modified: [last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on June 4, 2020 by Marie Benz MD FAAD
