
03 Feb UCSB Scientists Adapt Smartphone/Lab App to Cheaply and Accurately Detect COVID
MedicalResearch.com Interview with:

Dr. Mahan
Michael J. Mahan Ph.D
Professor
Dept of Molecular, Cellular, and Developmental Biology
University of California, Santa Barbara, CA 93106-9625
MedicalResearch.com: What is the background for this study?
Response: A critical need exists in resource-poor settings for low-cost, low-tech, yet highly reliable and scalable testing for SARS-CoV-2 virus that is robust against circulating variants.
MedicalResearch.com: What type of additional device to collect saliva would be required?
Response: The patient spits into a sterile collection cup.
Saliva samples are collected and the custom-built app uses a smartphone’s camera to measure a chemical reaction and determines an accurate diagnosis in 25 minutes
Image Credit: Courtesy Photo
MedicalResearch.com: What are the main findings?
Response: A smartphone-based COVID and influenza diagnostic was developed that is among the most rapid, sensitive, affordable, and scalable tests known. It can be readily adapted for other pathogens with pandemic potential including novel COVID variants and influenza.
MedicalResearch.com: What should readers take away from your report?
Response: The app and lab kit that uses a smartphone’s camera to measure a chemical reaction and determines a diagnosis in 25 minutes is as sensitive as PCR tests — at a fraction of the time and cost.
The lab kit can be produced for less than $100, and it requires little more than a smartphone, a hot plate, and LED lights. The screening tests cost less than $7 each versus $10 to $20 per rapid antigen test and $100 to $150 per PCR test.
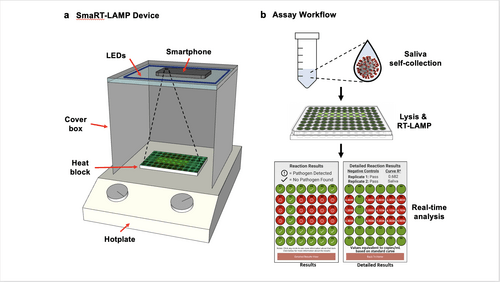
Device and Workflow Caption:
(a) LEDs are affixed to the inside top of a cardboard box that covers a heat block on a hotplate; and the smartphone camera is directed towards the samples.
(b) Workflow: The smaRT-LAMP reaction mix is loaded onto a heat block, which initiates amplification. The smartphone app displays the results as “Pathogen Detected” red circle; or “No Pathogen Found” – green circle.
Image Credit: Courtesy Photo
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Future research will be focused on optimizing smaRT-LAMP for inexpensive home-based testing.
MedicalResearch.com: Is there anything else you would like to add?
Response: We have provided the app and technology open source and freely available to all to help reduce the world’s inequities.
Citation:
Heithoff DM, Barnes L, Mahan SP, et al. Assessment of a Smartphone-Based Loop-Mediated Isothermal Amplification Assay for Detection of SARS-CoV-2 and Influenza Viruses. JAMA Netw Open. 2022;5(1):e2145669. doi:10.1001/jamanetworkopen.2021.45669
[wysija_form id=”3″]
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on February 3, 2022 by Marie Benz MD FAAD
