02 Apr Breast Cancer: Long Term Followup of Delayed Single-Dose Operative Radiotherapy vs Longer multiple-dose Postop Radiation Course
Professor Jayant S Vaidya MBBS MS DNB FRCS PhD
Professor of Surgery and Oncology,
Scientific Director, Surgical Interventional Trials Unit
Division of Surgery and Interventional Science,
University College London
jayantvaidya@gmail.com
http://www.targit.org.uk
Brief description
Scientists at University College London in collaboration with centres worldwide, developed and tested a technique which delivers breast cancer radiotherapy in a single sitting and avoids daily sessions over weeks requiring multiple trips to the hospital.
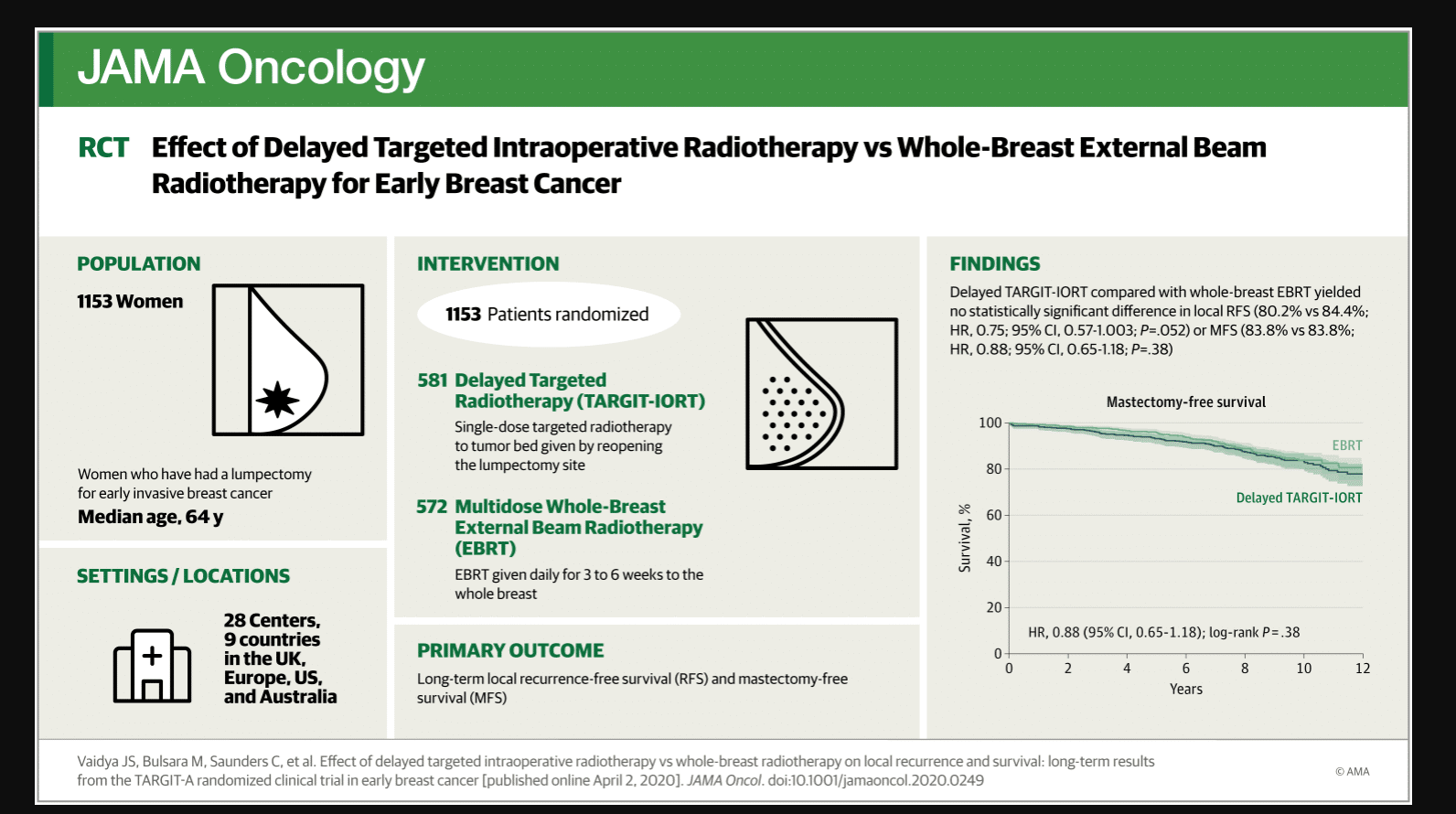
Study Aim: For early breast cancer, is 5-year local control with delayed second-procedure targeted intraoperative radiotherapy (TARGIT-IORT) non-inferior to whole breast postoperative radiotherapy (EBRT). With long-term term follow up, are the important outcomes comparable?
Findings: In this randomized controlled trial (n=1153), single-dose delayed TARGIT-IORT was not non-inferior to whole breast postoperative radiotherapy in terms of local recurrence at 5-years complete follow up. However, the more important long-term outcomes of mastectomy-free survival, distant disease-free survival, and overall survival with delayed TARGIT-IORT were similar to EBRT – median follow up 9 years.
Meaning: For early breast cancer, delayed second-procedure single-dose TARGIT-IORT, given by re-opening the lumpectomy wound, has similar long-term mastectomy-free and overall survival compared with whole breast postoperative radiotherapy. Although our preferred recommendation is to offer immediate TARGIT-IORT during lumpectomy, the use of single-dose delayed TARGIT-IORT as described in this paper will be of special relevance in those patients who have already had a lumpectomy. Travel for multiple hospital visits is greatly reduced, and it eases the pressure on radiotherapy departments.
More details:Background and Findings:
Conventional adjuvant radiotherapy for breast cancer given daily for several weeks is onerous and expensive. Some patients may be obliged to choose a mastectomy instead, and some may forgo radiotherapy altogether. In late 1990s, we proposed to test whether radiotherapy could be safely limited to the tumour bed. There were two trials that tested this hypothesis:
In the randomised trial of immediate TARGIT-IORT vs EBRT, 2298 patients who had been diagnosed with breast cancer by needle biopsy were enrolled. They were randomly allocated to receive to receive TARGIT-IORT immediately during their lumpectomy under the same anaesthetic, or conventional post-operative radiotherapy over several weeks. Its previously published initial results showed that TARGIT-IORT was as effective as EBRT, and the manuscript describing its long-term outcomes is currently under review.
In this randomised trial of delayed TARGIT-IORT vs EBRT, 1153 patients who had already had a lumpectomy for breast cancer were enrolled. They were randomly allocated to receive EBRT or delayed TARGIT-IORT given as a second procedure by reopening the lumpectomy wound. This paper describes the long-term outcomes of this trial of delayed IORT.
581 women were randomized to delayed TARGIT-IORT and 572 patients were randomized to EBRT. The results showed that delayed TARGIT-IORT was not noninferior to EBRT in terms of local recurrence at complete follow up of 5 years. However, long-term follow-up of median 9 years demonstrates no statistically significant difference in local recurrence-free survival, mastectomy-free survival, distant disease-free survival, or overall survival: these long-term outcomes with delayed TARGIT-IORT were similar to EBRT.
Takeaway Points:
For early breast cancer treated with lumpectomy, delayed second-procedure single-dose TARGIT-IORT given by re- opening the lumpectomy wound had 2.9% higher local recurrence at 5 years but there was no difference in the more important outcomes of long-term mastectomy-free, distant-disease-free and overall survival for up to 12 years, compared with EBRT. Although our preferred recommendation is to offer immediate TARGIT-IORT during lumpectomy wherever possible, the use of delayed TARGIT-IORT as described in this paper could be considered in those who have already had a lumpectomy.
This could be of special relevance during the current COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2.. This is because many patients who have already undergone breast-conserving surgery may have to wait for many weeks, or even longer, for their post-operative external-beam radiotherapy. Such patients can be reassured as part of an informed decision-making process, that they can be satisfactorily treated by delayed TARGIT-IORT, and that their long-term prognosis is essentially just as good. Travel and therefore viral exposure would be greatly reduced. Increasing the availability of this treatment would greatly ease the pressure on radiotherapy departments, since conventional radiotherapy for breast cancer forms such a large portion of a typical departmental workload. Precious machine-time and expertise would therefore be made available for many other purposes.
These results should enable more patients to take advantage of this treatment. TARGIT-IORT is already used as part of standard care in 300 centres in 38 countries. More details at www.targit.org.uk
Effect of Delayed Targeted Intraoperative Radiotherapy vs Whole-Breast Radiotherapy on Local Recurrence and Survival
Disclosures The trial was supported by funding from the Health Technology Assessment programme of the National Institute of Health Research, Department of Health, UK, and the UCL/UCLH Comprehensive Biomedical Research Centre. Some authors have received a research grant from Photoelectron Corp (1996-1999) and from Carl Zeiss for supporting data management at the University of Dundee (2004-2008) and has received honoraria and travel costs.
Citation:
JOIN OUR EMAIL LIST
[mailpoet_form id="5"]We respect your privacy and will never share your details.
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on April 2, 2020 by Marie Benz MD FAAD