
31 Mar Does evergreening contribute to the prescribing pattern of citalopram and escitalopram to U.S. Medicaid and Medicare patients?

Parita K. Ray
Parita K. Ray
Medical Student
Department of Medical Education
Geisinger College of Health Sciences
Scranton, PA 18509
MedicalResearch.com: What is the background for this study?
- Citalopram and escitalopram are two of the most commonly prescribed antidepressants in the U.S. and are widely used for treating major depressive disorder (MDD) and generalized anxiety disorder (GAD), along with various off-label indications. While both medications share similar mechanisms of action, escitalopram was introduced as a purified version of citalopram’s active enantiomer. Despite little evidence showing a clear superiority of escitalopram over citalopram in efficacy or tolerability, prescribing trends appear to favor escitalopram, raising concerns about “evergreening”—a practice where pharmaceutical companies promote newer formulations of existing drugs to extend market exclusivity. Prior research has shown variations in the prescription rates of psychotropic medications across states and within specific populations, particularly among Medicaid and Medicare patients. However, little is known about the long-term prescribing patterns of citalopram versus escitalopram in these populations and whether these trends reflect potential evergreening practices.
MedicalResearch.com: What are the key points of your research?
- The study examined national and state-level trends in citalopram and escitalopram prescriptions among Medicaid and Medicare patients. It tested the hypothesis that citalopram prescriptions have declined over time, with a concurrent increase in escitalopram prescriptions, consistent with evergreening.
- Our findings revealed that the overall prescribing rate of escitalopram increased significantly, while the prescribing rate of citalopram declined. From 2015 to 2019, we saw -26.0% decreasing prescriptions for citalopram and +41.2% increasing rate of prescriptions for escitalopram. This shift was especially pronounced in Medicare beneficiaries, where the prescribing rate of escitalopram showed an incline over the study period. In contrast, citalopram prescriptions decreased during the same time frame.Our study also highlighted that the shift from citalopram to escitalopram was not only influenced by clinical guidelines and FDA recommendations but also reflected changes in prescriber preferences and patient outcomes. In conclusion, our research shows a clear pattern toward the increased use of escitalopram over citalopram in the Medicaid and Medicare populations, likely driven by pharmacoeconomic, clinical and demographic factors.
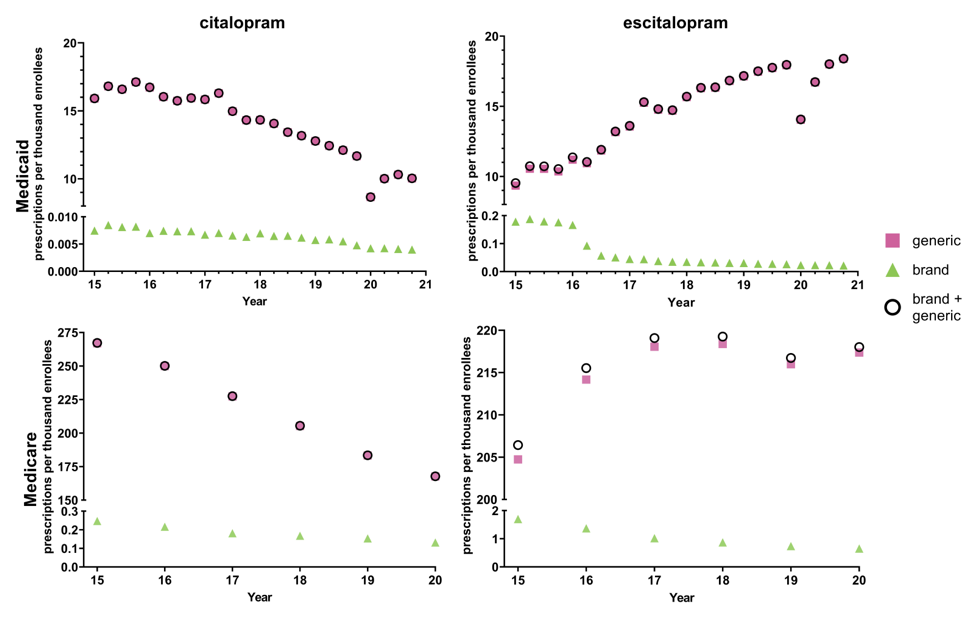
Figure 1. National Medicaid and Medicare population-corrected prescriptions for generic and brand name formulations of citalopram and escitalopram 2015-2020
MedicalResearch.com: Was the outcome compatible with your premise or were the results surprising?
- We hypothesized that escitalopram prescriptions would increase significantly compared to the citalopram consistent with the concept of evergreening and supported previous findings on industry-driven prescribing patterns. Our findings clearly supported this, showing a rise in escitalopram use from 32% to 57% over five years, while citalopram prescriptions decreased.
- However, regional variations were more pronounced than expected, with the Northeast showing a higher preference for escitalopram than the South. The difference between the lowest and highest prescribing states was about four-fold for escitalopram and five-fold for citalopram in Medicare. The differences were even larger among Medicaid patients with eleven-fold for citalopram and nineteen-fold for escitalopram. Treatment guidelines for psychiatric disorders do not differentiate between whether the patient resides in West Virginia versus Washington DC.
- Overall, the results were consistent with our hypothesis but the pronounced state-level differences, although consistent with prior studies with other drugs, were a surprising aspect of the data.
MedicalResearch.com: What further questions remain to be answered? What further research is indicated?
- Our study highlights trends in the use of escitalopram and citalopram, but further study is needed to explore individual-level factors that influence prescribing patterns. Understanding the reasons behind provider preferences for escitalopram over citalopram would provide valuable insight into clinical decision-making. Additionally, future research should focus on assessing patient outcomes and adverse effects associated with these medications across different regions to gain a deeper understanding of the impact of prescribing trends. It would also be beneficial to investigate how evolving clinical guidelines and insurance coverage policies influence prescribing practices for SSRIs.
Citation:
Ray PK, et al. Patterns in (es)citalopram prescriptions to Medicaid and Medicare patients in the United States: The potential effects of evergreening. Frontiers of Psychiatry 2025; https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2025.1450111/full#supplementary-material
References
Cavanah LR, Goldhirsh JL, Huey LY, Piper BJ. National patterns of paroxetine use among U.S. Medicare patients from 2015-2020. Frontiers of Psychiatry. 2024; 15:1399493.
Centers for Medicare & Medicaid Services. Data.CMS.gov. Medicare Part D prescribers. Available from: https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers
Dwivedi G, Hallihosur S, Rangan L. Evergreening: A deceptive device in patent rights. Technology in Society2010;32(4):324–30. Available from: https://www.sciencedirect.com/science/article/pii/S0160791X1000076X
Kane SP. The Top 300 of 2013, ClinCalc DrugStats Database, Version 2024.01. 2024 [cited 2024 Jun 16]. ClinCalc. Available from: https://clincalc.com/DrugStats
Kane SP. The Top 300 of 2020, ClinCalc DrugStats Database, Version 2024.01. 2024. ClinCalc. Available from: https://clincalc.com/DrugStats
Medicaid. Data.CMS.gov. [cited 2022 Apr 25]. State drug utilization data. Available from: https://www.medicaid.gov/medicaid/prescription-drugs/state-drug-utilization-data/index.html
Milne RJ, Goa KL. Citalopram. Drugs 1991;41(3):450–77. Available from: https://doi.org/10.2165/00003495-199141030-00008
Research C for DE and. FDA. FDA; 2019 [cited 2024 Jun 16]. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-revised-recommendations-celexa-citalopram-hydrobromide-related
Waugh J, Goa KL. Escitalopram. CNS Drugs 2003;17(5):343–62.
- If you or someone you know is struggling or in crisis, help is available. Call or text 988 or chat at org. To learn how to get support for mental health, drug or alcohol conditions, visit FindSupport.gov. If you are ready to locate a treatment facility or provider, you can go directly to FindTreatment.govor call 800-662-HELP (4357).
- US. veterans or service members who are in crisis can call 988 and then press “1” for the Veterans Crisis Line. Or text 838255. Or chat online.
- The Suicide & Crisis Lifeline in the U.S. has a Spanish language phone line at 1-888-628-9454 (toll-free).
—————
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition.
Some links may be sponsored.
Eminent Domains Inc and MedicalResearch.com are not responsible for the content of links. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on March 31, 2025 by Marie Benz MD FAAD
