01 Dec Gene Therapy for Mitochondrial Eye Disease Developed
MedicalResearch.com Interview with:
Daniel Maloney, Ph.D
Farrar Lab
Smurfit Institute of Genetics
Trinity College Dublin
MedicalResearch.com: What is the background for this study? Would you briefly describe the condition of Dominant optic atrophy?
Response: Dominant Optic Atrophy (DOA) is a progressive blinding disorder that affects roughly 1:10,000 to 1:30,000 people. It is primarily caused by mutations in the OPA1 gene, which plays a pivotal role in the maintenance of the mitochondrial network. There is currently no way to prevent or cure DOA. We sought to build upon previous work to test if OPA1 could be delivered as a potential gene therapy intervention.
MedicalResearch.com: What are the main findings?
Response: To investigate the potential of two OPA1 isoforms as gene therapy interventions we utilised two models, an in vivo model of general mitochondrial dysfunction brought on by a chemical insult, and an in vitro model using DOA patient derived fibroblasts. Using AAV viruses to deliver either OPA1 isoform 1 or 7, we were able to protect visual acuity in the in vivo model, as well as boosting mitochondrial function in the patient derived fibroblasts. Intriguingly we observed differences between the two isoforms in their efficacy in these two models.
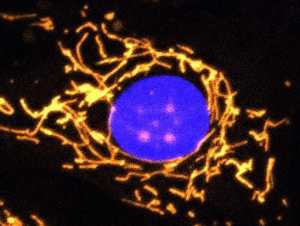
A fluorescent microscope image with mitochondria highlighted in gold. This healthy cell shows a highly elaborate and well-connected network of mitochondria.
Credit: Professor Jane Farrar and Dr Daniel Maloney, Trinity College Dublin

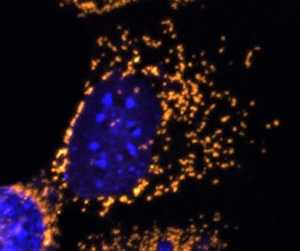
A fluorescent microscope image with mitochondria highlighted in gold. This cell completely lacks OPA1 protein and shows fragmented mitochondria.
Credit: Professor Jane Farrar and Dr Daniel Maloney, Trinity College Dublin
MedicalResearch.com: What should readers take away from your report?
Response: We have shown that there is potential to use AAV delivered OPA1 based gene therapies for modulating cellular bioenergetics both in vitro and in vivo. We build upon previous work by demonstrating that OPA1 based gene therapies could potentially provide benefit not only for DOA patients but also in principle for more general mitochondrial dysfunction.
MedicalResearch.com: What recommendations do you have for future research as a result of this study?
Response: Our study highlights the importance of tightly regulating the expression of the OPA1 protein in order to maximise any potential benefit. Future work will need to focus on how to correctly regulate the levels of OPA1 to ensure that suitable levels are consistently maintained. There are also some interesting questions relating to the differing functionality of isoform 1 and 7, and future work will be needed to fully explore if each isoform has unique strengths as potential therapeutic entities.
MedicalResearch.com: Is there anything else you would like to add? Any disclosures?
Response: The study involved our team in the School of Genetics and Microbiology, Trinity College Dublin and clinical teams in the Royal Victoria Eye and Ear and Mater Misericordia Hospitals, Dublin. The study was supported by Science Foundation Ireland, Fighting Blindness Ireland, Health Research Board and Health Research Charities Ireland. We are entering the era of gene therapy, with a number of FDA/EMA approved therapies becoming available, including one for a different inherited eye condition (LuxturnaTM). Undoubtedly we will see approvals for additional effective gene therapies over the next few years – there are positive times ahead for the field and most importantly for patients.
Citation:
Maloney Daniel M., Chadderton Naomi, Millington-Ward Sophia, Palfi Arpad, Shortall Ciara, O’Byrne James J., Cassidy Lorraine, Keegan David, Humphries Peter, Kenna Paul, Farrar Gwyneth Jane
Optimized OPA1 Isoforms 1 and 7 Provide Therapeutic Benefit in Models of Mitochondrial Dysfunction
Frontiers in Neuroscience VOLUME=14 YEAR=2020 PAGES=1213
https://www.frontiersin.org/article/10.3389/fnins.2020.571479 DOI=10.3389/fnins.2020.571479 ISSN=1662-453X
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on December 1, 2020 by Marie Benz MD FAAD
