
16 Jan SING IMT for Late-Stage, Age-Related Macular Degeneration from Samsara Vision
MedicalResearch.com Interview with:
Prof Alfonso Savastano
Ospedale Generale Regionale
“F. Miulli” – Acquaviva delle Fonti (BA) and
Libera Università Mediterranea Degennaro (L.U.M.)- Casamassima (BA)
MedicalResearch.com: What is the background for this study?
Response: The SING IMT® (Smaller-Incision New-Generation Implantable Miniature Telescope) is a novel, intraocular device for people blinded in the central vision by late-stage, age-related macular degeneration (AMD), the leading cause of unpreventable blindness. It is approved for use in CE referenced countries and under investigation in the United States.
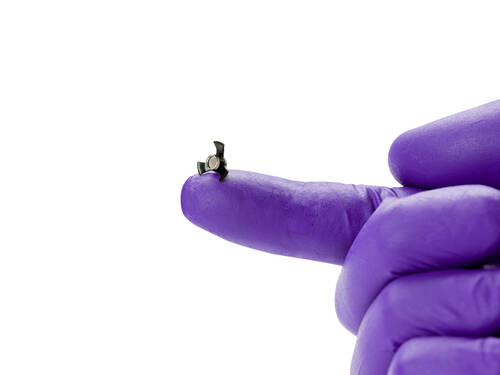
SING IMT for Late-Stage, Age-Related Macular Degeneration from Samsara Vision
Our study evaluated the intermediate-term visual and safety outcomes of the SING IMT in patients (n=35) 55 years and older at 6 months post-surgery and found that SING IMT implantation improved distance and near vision, with an expected impact on the corneal endothelium cell density and manageable safety outcomes. Key findings include:
- At six months post-surgery, at least 1-, 2-, and 3-line gains in best-corrected distance (BCDVA) were achieved in 97.1 percent, 68.6 percent and 51.4 percent of operated eyes, respectively
- The percentage of patients able to read at near distance increased from 28.6 percent at baseline to 97.1 percent at six months.
- The study also found that corrected near visual acuity was also significantly improved by ⁓3 lines at 6 months post-surgery.
- The mean (SD) change from baseline in corneal endothelial cell density (ECD) at six months in operated eyes was -280.7 (315.9) cells/mm2 (-11.4 %). This is a result similar to that seen with standard cataract surgery.
- The most frequent adverse event was corneal edema, and all cases were resolved with topical medications.
MedicalResearch.com: Is the ‘telescope’ implanted in one or both eyes? How does it work?
Response: The SING IMT is implanted monocularly and the other eye is left as-is to preserve peripheral vision. Post-surgery, patients participate in required vision rehabilitation where they learn to use their new vision, practicing skills while stationary and while moving that relate to visual abilities, reading, writing, visual motor integration, and mobility. One of the best indicators of functional vision is the ability to read and it was gratifying to see nearly all our patients reading at 6-months. It’s a remarkable change because these patients lose their ability to see faces, watch TV, read and participate in the activities of daily living when their central vision is lost.
The SING IMT is a second generation device whose design improves upon the earlier iteration by being easier and quicker to insert, surgically, with fewer sutures and a shorter recovery time. It has a Galilean design, based on ultra-precision, wide-angle micro-optics that, in combination with the optics of the cornea, create a telephoto system that magnifies objects in view. How it works is that images seen in central vision are magnified 2.7x and projected onto healthy photoreceptors surrounding the macula in the back of the eye, reducing the impact of the AMD “blind spot” in central vision and allowing patients to see images that may have been unrecognizable before. The central scotoma is not eliminated or cured, but patients can better see “around” it as images are projected onto healthier parts of retina undamaged by AMD.
MedicalResearch.com: What should readers take away from your report?
Response: While there are other existing treatments for AMD that may slow down progression of the disease, none are a cure or are proven to improve vision in those people who have progressed to an advanced form of the disease. This study should provide both providers and patients with additional confidence in the safety and efficacy of the SING IMT as an important option for patients with late-stage AMD.
MedicalResearch.com: What recommendations do you have for future research as a result of this study?
Response: We will continue to follow these patients, and others, to monitor efficacy and safety outcomes over time to confirm the long-term results from SING IMT. The SING IMT is also under clinical investigation in the United States with results expected in 2025.
The SING IMT is indicated for use in age-related macular degeneration patients diagnosed with cataract(s). The SING IMT is currently being evaluated for pseudophakic patients who have previously undergone cataract surgery and have in intraocular lens in place.
No disclosures
Citation:
- Smaller-incision new-generation implantable miniature telescope in late-stage age-related macular degeneration: 6 month outcomes
Toro, Mario Damiano et al. Heliyon, Volume 11, Issue 1, e41116
https://www.cell.com/heliyon/fulltext/S2405-8440(24)17147-2
—————-
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition.
Some links may be sponsored. Products are not warranted or endorsed.
Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on January 16, 2025 by Marie Benz MD FAAD
