Author Interviews, Genetic Research, Lancet, Ophthalmology / 26.02.2025
Gene Therapy Reversed Blindness in 11 Children with Congenital Disease
MedicalResearch.com Interview with:
Professor Michel Michaelides BSc MB BS MD(Res) FRCOphth FACS
Professor of Ophthalmology and Consultant Ophthalmic Surgeon
UCL Institute of Ophthalmology and Moorfields Eye Hospital
MedicalResearch.com: What is the background for this study?
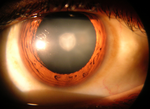
- Leber congenital amaurosis (LCA) is a rare inherited retinal disease (IRD) that causes the degeneration of the cone and rod cells in the retina. It is characterized by severe vision loss at birth/early infancy and is one of the commonest causes of legal blindness in childhood. There is a wide range of severity at birth/early infancy, ranging from light perception to useful central vision; with variable rate of progression over time, LCA is estimated to occur in about 1 in 33,000 people. There are more than 30 genes that cause LCA.
- LCA associated with genetic deficiency of AIPL1 is one of the most severe forms of LCA – resulting in profound visual loss from birth (often light perception only) and rapidly progressive retinal degeneration.
- The study sought to evaluate whether early intervention by gene supplementation therapy in children with LCA associated with AIPL1 was safe and could improve outcomes.












 Another name for LASIK is Laser-Assisted In Situ Keratomileusis, a popular vision correction surgery that has helped millions achieve more precise vision. It involves using advanced laser technology to modify the cornea to let light reach the eye and be correctly focused onto the retina. This reshaping process significantly improves vision for those with refractive defects, including astigmatism, farsightedness, and nearsightedness. If you've been considering options like
Another name for LASIK is Laser-Assisted In Situ Keratomileusis, a popular vision correction surgery that has helped millions achieve more precise vision. It involves using advanced laser technology to modify the cornea to let light reach the eye and be correctly focused onto the retina. This reshaping process significantly improves vision for those with refractive defects, including astigmatism, farsightedness, and nearsightedness. If you've been considering options like
 LASIK is an excellent option for people who have to wear glasses or contacts and don't want to do that anymore. Before going in for a consultation, it's good to learn as much as possible about it. Here are some answers, including ones if you have
LASIK is an excellent option for people who have to wear glasses or contacts and don't want to do that anymore. Before going in for a consultation, it's good to learn as much as possible about it. Here are some answers, including ones if you have 




 MedicalResearch.com: What is the mission of
MedicalResearch.com: What is the mission of