06 Oct NEJM: Trial of IVIG for Autoimmune Disorder Dermatomyositis
MedicalResearch.com Interview with:
Rohit Aggarwal, MD, MS
Rheumatology, Professor of Medicine
Medical Director, Arthritis and Autoimmunity Center
Sub-Specialty Education Coordinator
Division of Rheumatology Department of Medicine
University of Pittsburgh
MedicalResearch.com: What is the background for this study?

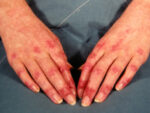
One example of Dermatomyositis: DermNet image
Response: Dermatomyositis is a rare autoimmune inflammatory disease that affects muscles and skin, although muscular forms without skin symptoms and vice versa are also seen. The exact etiology of the disease is not known but is thought to be immune-mediated with many patients having highly specific autoantibodies. There is no cure for dermatomyositis, but several types of treatment have been successfully used in the last years including different kinds of immunosuppressants (e.g. steroids) and intravenous immune globulins (IVIG) to improve the patient’s condition. So far, none of these treatments was approved for use in dermatomyositis based on large, randomized, placebo-controlled trials. Their effectiveness was mainly deduced from clinical experience and from small clinical trials. The ProDERM study was the first large, pivotal, randomized placebo-controlled trial to evaluate the efficacy and safety of intravenous immune globulin (IVIG) in dermatomyositis patients.
Response: What are the main findings and side effects of the IVIG infusions?
Response: In the first period which was placebo-controlled, randomized and double-blind (neither physicians and nurses nor patients knew the allocated treatment) significantly more patients achieved the primary endpoint and responded to IVIG (octagam 10% – Octapharma) treatment than patients treated with placebo. The primary endpoint was minimal improvement based on an approved criterion. The results also showed that this was true for different categories of response (mild, moderate, and major). In addition, more patients showed at least moderate and major improvement when treated with IVIG than those treated with placebo. Note that the study was not powered for secondary endpoints. Typical skin symptoms also improved after IVIG treatment.
In the open-label extension period, all patients received IVIG infusions and those who were on placebo in the first period showed similar improvement upon IVIG treatment as patients who had already received IVIG in the first period.
Side effects of IVIG infusions were seen in 65% of patients and most side effects were well-known events for IVIG infusions and of mild intensity, including headache, fever, and nausea. There were also 6 thromboembolic events (TEE) in 5 patients that were assessed as being related to the IVIG infusions. Dermatomyositis patients are known to be more prone to experience TEEs due to the inflammatory status of the disease and the decreased mobility triggered by their muscle weakness. IVIG is also known to cause TEEs, especially when given at high doses, which was the case in this study, and thus we looked specifically for this type of side effects. Due to occurring TEEs the allowed maximum infusion rate was reduced during the study which entailed a reduction in the incidence of TEEs.
MedicalResearch.com: What should readers take away from your report?
Response: The ProDERM study demonstrated that IVIG is an effective treatment for dermatomyositis improving muscle as well as skin symptoms. The results of the study led to the approval of dermatomyositis as an indication for octagam 10% (IVIG-Octapharma) which is, therefore, the first FDA-approved treatment for this condition based on a large phase 3 clinical trial. Active DM was also approved as indication for Octagam 10% in several EU countries as well as Canada based on the ProDERM trial results.
The higher risk of TEEs in these patients due to their disease as well as IVIG treatment should be considered and a low infusion rate according to the package insert should be used when treating dermatomyositis patients with IVIG.
MedicalResearch.com: What recommendations do you have for future research as a results of this study?
Response: The first approval of dermatomyositis as an indication for IVIG was a major achievement for the DM community as it leads to a pathway for future clinical trials and approvals.
However, work in DM has just started and we need do a lot on IVIG as well as DM to provide safe and effective treatment for our patients.
Further research should be done on dosing, frequency and steroid sparing effect. In the ProDERM study 8 patients who improved on IVIG treatment and then were stable in their disease condition could successfully reduce their IVIG dosing in the open label period. As this was not part of the secondary or exploratory endpoints of the study but at the discretion of the treating physician, more research is needed on possible IVIG dose reduction in these patients once they have improved and are stable. Further concomitant immunosuppressive DM therapy which needed to be kept stable during the placebo-controlled period could be reduced during the open-label period and about 20% of patients could successfully reduce or stop their steroid dosing. Here again, controlled studies are warranted to explore the steroid-sparing effect of IVIG treatment in more depth.
Additionally, autoantibodies are important biomarkers in patients with inflammatory disease and more information is needed if the response to IVIG treatment is dependent on the autoantibody status of the patient.
More research into IVIG efficacy in pediatric patients with inflammatory myopathies is also highly needed as are trials in other forms of inflammatory myopathies, such as immune-mediated necrotizing myopathy).
Disclosures: This study was sponsored by Octapharma PPG
Citation:
Trial of Intravenous Immune Globulin in Dermatomyositis
Rohit Aggarwal, M.D., Christina Charles-Schoeman, M.D., Joachim Schessl, M.D., Zsuzsanna Bata-Csörgő, M.D., Dr.Sc., Mazen M. Dimachkie, M.D., Zoltan Griger, M.D., Ph.D., Sergey Moiseev, M.D., Chester Oddis, M.D., Elena Schiopu, M.D., Jiri Vencovský, M.D., Dr.Sc., Irene Beckmann, Elisabeth Clodi, Ph.D., et al., for the ProDERM Trial Group†
October 6, 2022 N Engl J Med 2022; 387:1264-1278
DOI: 10.1056/NEJMoa2117912
https://www.nejm.org/doi/full/10.1056/NEJMoa2117912
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on October 6, 2022 by Marie Benz MD FAAD
