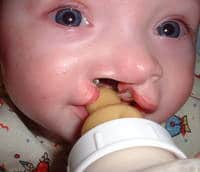
02 Oct Mechanism Underlying Cleft Lip & Palate Identified in Mouse Model
MedicalResearch.com Interview with:
Manvendra K Singh PhD
Program in Cardiovascular and Metabolic Disorders, Duke-NUS Medical School
National Heart Research Institute, National Heart Center
Singapore, Singapore
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Craniofacial and cardiovascular abnormalities are the most common defects, contributing to more than one-third of the congenital diseases. Proper formation of these structures involves intricate processes such as proliferation, migration, and differentiation of neural crest cells (NCCs). Functional defects in NCCs result in craniofacial malformations, including cleft lip and/or cleft palate. Many transcription factors, chromatin remodelling factors, non-coding RNA and signalling molecules have been implicated in impaired neural crest development that result in cardio-craniofacial syndromes. However, the cell-autonomous role of splicing regulators in neural crest biology remains unclear and warrants further investigation.
MedicalResearch.com: What are the main findings? How is this mechanism affected by folic acid?
Response: In the present study, we demonstrate that splicing factor Rbfox2 is expressed in the NCCs. Genetic tissue-specific deletion of Rbfox2 in NCCs leads to cleft palate and defects in craniofacial bone development. RNA-Seq analysis demonstrated that Rbfox2 regulates splicing and expression of numerous genes essential for neural crest/craniofacial development. We further observed that Rbfox2-TGF-β-Tak1 signalling axis is deregulated by Rbfox2 deletion in NCCs. Furthermore, restoration of TGF-β signalling by Tak1 overexpression can rescue the proliferation defect seen in Rbfox2 mutants. We also identified a positive feedback loop in which TGF-β signalling promotes expression of Rbfox2 in NCCs. Together, our results reveal a highly regulated Rbfox2-dependent splicing and transcriptional program that modulates cranial neural crest development.
Several studies have shown that folic acid may have a protective effect on cleft palate and neural tube defects. However, we have not performed any experiment yet to determine whether folic acid had any protective effect on cleft palate present in Rbfox2 mutants.
MedicalResearch.com: What should readers take away from your report?
Response: We have discovered the underlying splicing and molecular changes associated with the craniofacial abnormalities including cleft palate. This will help us to better understand and treat the congenital defects in future.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Here, we not only demonstrate that Rbfox2 is essential for the development of tissues derived from NCCs, but also uncover over 100 Rbfox2-dependent splicing events that occur during neural crest development. We will further characterized the expression and splicing patterns of some of the newly identified targets from our RNA-Seq screen.
Citation:
Neural crest-specific deletion of Rbfox2 in mice leads to craniofacial abnormalities including cleft palate
Dasan Mary Cibi, Masum M Mia, Shamini Guna Shekeran, Lim Sze Yun, Reddemma Sandireddy, Priyanka Gupta, Monalisa Hota, Lei Sun, Sujoy Ghosh, Manvendra K Singh
Elife. 2019 Jun 26;8. pii: e45418. doi: 10.7554/eLife.45418.
[wysija_form id=”3″]
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on October 2, 2019 by Marie Benz MD FAAD

