13 Mar COVID-19: Research and Development on Therapeutic Agents and Vaccines
MedicalResearch.com Interview with:
Cynthia Liu, Ph.D.
Manager, Scientific Information
CAS, a division of the American Chemical Society
Columbus, OH 43210
MedicalResearch.com: What is the background for this study?
Response: The outbreak of COVID-19 caused by the new virus SARS-CoV-2 has overwhelmed the health systems in many countries and been declared by WHO as a pandemic which will continue to affect global public health and world economy. This threat calls for an intensified effort in the development of therapeutic agents and vaccines. CAS is a not-for-profit division of the American Chemical Society that specializes in scientific information solutions. Our team includes hundreds of scientists that build a global data collection of curated scientific content from both journal articles and patent applications as well as chemical and biological substance collections. With this report, our team hopes to support the efforts of R&D organizations seeking to address this crisis by providing an up-to-date overview of recent relevant publications and insight into potential therapeutic agents, including both small molecules and biologics.
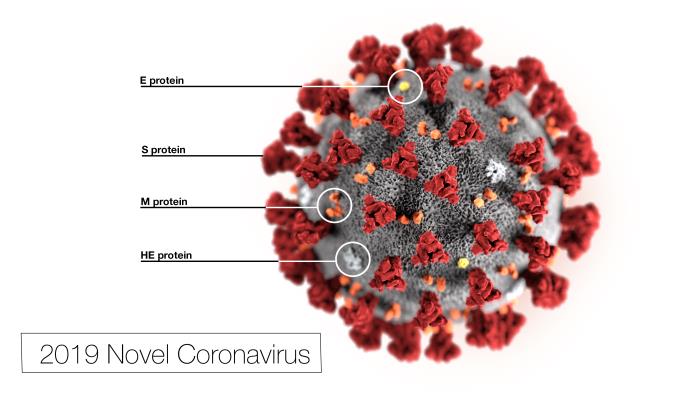
This illustration, created at the Centers for Disease Control and Prevention (CDC), reveals ultrastructural morphology exhibited by the 2019 Novel Coronavirus (2019-nCoV). Note the spikes that adorn the outer surface of the virus, which impart the look of a corona surrounding the virion, when viewed electron microscopically. In this view, the protein particles E, S, M, and HE, also located on the outer surface of the particle, have all been labeled as well. This virus was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China.
MedicalResearch.com: What are the main findings?
1) Scientists worldwide have published over 500 relevant journal articles in the past two months; a very fast pace. Some of these publications have greatly facilitated the drug and vaccine development efforts.
2) One key focus of current research is identifying the existing antiviral drugs that may be repurposed for COVID-19 treatment, and this report includes a list of small molecule drugs s have been identified as potential candidates.
3) We examined over 500 patents on related human coronaviruses such as SARS-CoV and MERS-CoV that pointed to some strategies used in the development of biologic therapeutics including antibodies, cytokines and RNA-based therapeutic agents, which may be applicable to the development of biologics for COVID-19.
4) About 190 patents related to the development of vaccines for SARS and MERS have also been identified and are made available in our report. The strategies covered in these patents, which are also being used in the development of COVID-19 vaccines, range from attenuated virus vaccines and protein-based vaccines to virus-like particle vaccines and mRNA-based vaccines.
MedicalResearch.com: What should readers take away from your report?
Response: Research activity around COVID-19 is already rapidly accelerating to address this global threat. This work can be informed by the extensive body of published research on related viruses such as SARS-CoV and MERS-CoV and the treatments that have proven successful with other virus-induced infections. One of the most efficient ways to expedite cures for new diseases is to draw on existing knowledge in the related scientific areas. That is the primary focus of this report and the overall work of CAS.
MedicalResearch.com: What are the next steps in treatment and vaccine research?
Response: Drug repurposing will likely be the next step in short-term. Over the long run, small molecules and biologics with potential applications to any RNA virus-induced diseases may be discovered and screened, and those with broad-spectrum antiviral effects will be more closely examined. Cutting edge technologies such as mRNA vaccines are being used in the development of vaccines for COVID-19 and other related diseases, and are speeding up the vaccine development. However, once developed, it will still take time to bring any COVID-19 vaccine to market because of necessary clinical trials.
MedicalResearch.com: Is there anything else you would like to add?
Response: The mission of the American Chemical Society, and CAS as a division, is “Improving people’s lives through transforming power of chemistry”. This report is an initial step in our endeavors to support the scientific community in developing drugs and vaccines for COVID-19. If there are other ways that CAS can support research teams actively working on this problem with things such as expert search support, analytics, custom data sets, consulting or product access, please reach out to us as we may be able to offer these on a gratis basis, as our capacity allows, to support this important work.
About CAS
CAS, a division of the American Chemical Society specializing in scientific information solutions, partners with R&D organizations globally to provide actionable insights that help them plan, innovate, protect their innovations, and predict how new markets and opportunities will evolve. Scientific researchers, patent professionals and business leaders around the world across commercial, academic and government sectors rely on our solutions and services to advise discovery and strategy. Leverage our unparalleled content, specialized technology, and unmatched human expertise to customize solutions that will give your organization an information advantage. With more than 110 years’ experience, no one knows more about scientific information than CAS. Learn more at www.cas.org.
Citation:
ACS Cent. Sci. 2020, XXXX, XXX, XXX-XXX
Publication Date:March 12, 2020
https://doi.org/10.1021/acscentsci.0c00272
JOIN OUR EMAIL LIST
[mailpoet_form id="5"]We respect your privacy and will never share your details.
Last Modified: [last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on March 13, 2020 by Marie Benz MD FAAD
