
14 Jan New study finding $14.2 million in undisclosed conflicts of interest in the “bible” of psychiatry (DSM-5-TR) published in the British Medical Journal
MedicalResearch.com Interview with:

Lauren C. Davis, MBS
Department of Medical Education
Geisinger Commonwealth School of Medicine
Scranton, PA 19409
MedicalResearch.com: What is the background for this study?
Response: Financial conflicts of interest (COIs) resulting from ties between academia and industry have been under scrutiny for their potential to hinder the integrity of medical research. COIs can lead to implicit bias, compromise the research process, and erode public trust (1-6). The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM), standardizes symptom criteria and codifies psychiatric disorders. This manual contributes to the approval of new drugs, extensions of patent exclusivity, and can influence payers and mental health professionals seeking third-party reimbursements. Given the implications of the DSM on public health, it is paramount that it is free of industry influence. Previous research has shown a high prevalence of industry ties among panel and task force members of the DSM-IV-TR and DSM-5, despite the implementation of a disclosure policy for the DSM-5 (7,8). This study (9) determined the extent and type of COIs received by panel and task-force members of the DSM-5-TR (2022) (10). As the DSM-5-TR did not disclose COI, we used the Center for Medicare and Medicaid Services Open Payments (OP) database (11) to quantify them.
 MedicalResearch.com: What are the main findings?
MedicalResearch.com: What are the main findings?
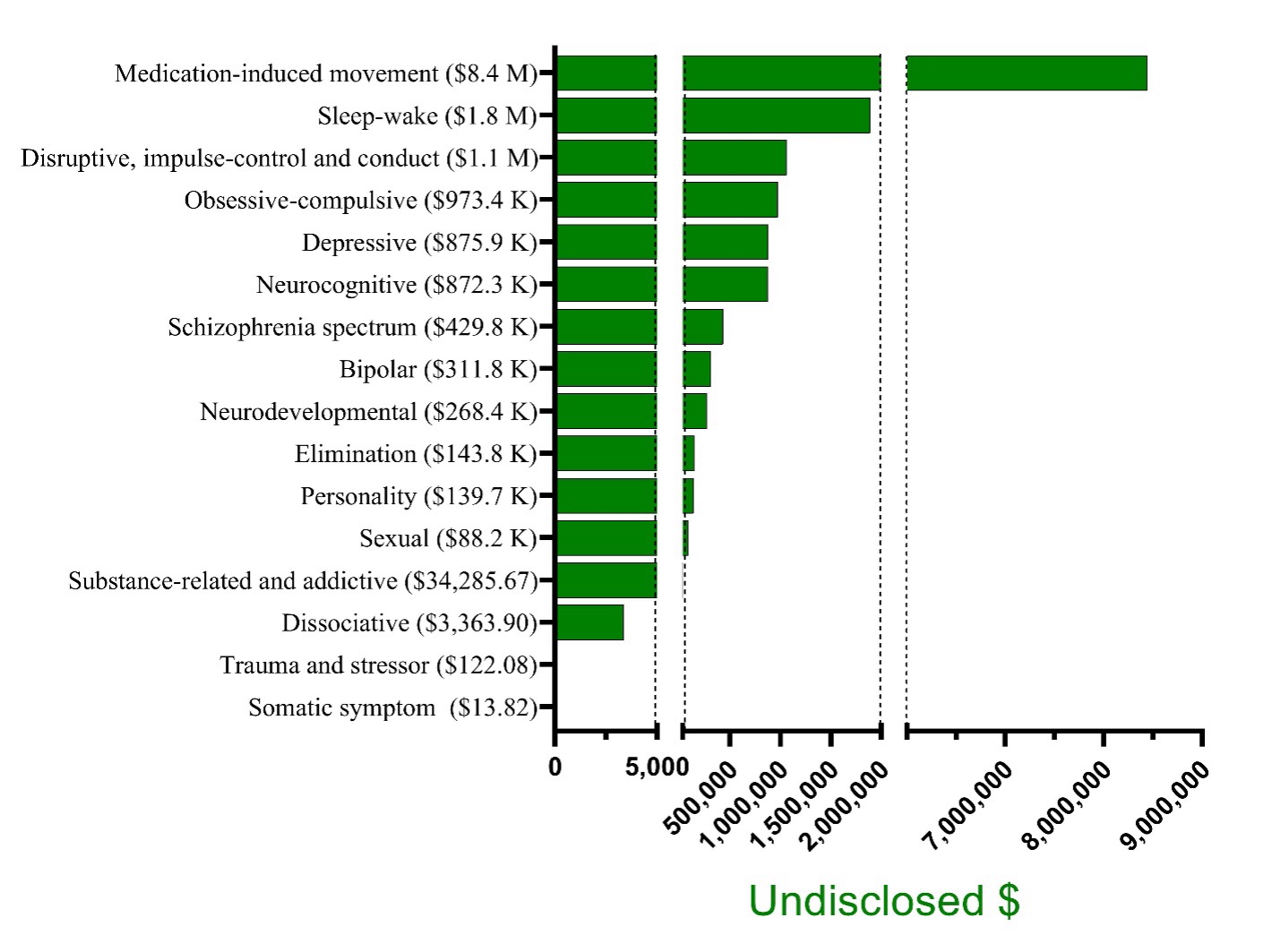
Response: Of the 168 individuals who served as either DSM panel or task force members, 92 met inclusion criteria of being US-based physicians (MD or DO) who could be included in the US OP database (11). Of the 92 individuals meeting inclusion criteria, 55 authors (59.8%) received payments from industry. Collectively these individuals received a total of $14.24 million with 70.6% for research, 8.3% for consulting, and 4.8% for travel. Furthermore, one-third of the task force members had payments reported in OP. Out of the twenty total DSM-5-TR work groups and task force, five work groups consisted of > 75% members that received industry compensation.
Further examination of the work groups revealed substantial differences in undisclosed compensation (Figure, K = x1,000; M = x1,000,000). Although anxiety disorders, feeding andeating disorders, gender dysphoria, and paraphilic disorder work group members received no renumeration, the amount received by other workgroups was considerable (Obsessive-compulsive disorders = $973,851, Disruptive disorders = $1,059,910, Sleep-week disorders = $1,892,431, and Medication-induced movement disorders = $8,443,468).
MedicalResearch.com: What should readers take away from your report?
Response: The DSM has been referred to as the ‘bible” of psychiatry and industry influence over the development of this diagnostic guideline can have a profound effect on public health (e.g., by broadening diagnostic categories and influencing what medications will be prescribed and covered by insurance). Thus, it is critical that this psychiatric taxonomy is free of industry influence, or even the appearance of such influence. There is an abundance of research documenting the impact of financial conflicts of interest on medical literature, including randomized clinical trials, meta-analyses, and clinical diagnostic and practice guidelines (12-16). Such research has consistently shown that conflicts of interest lead to subtle but impactful pro-industry thinking and conclusions (17).
Our finding of a $14.2 million in undisclosed industry compensation from pharmaceutical companies to the DSM-5-TR developers is cause for concern.
MedicalResearch.com: What recommendations do you have for future research as a results of this study?
Response: A members of our research team made the following recommendations in 2012: 1) Individuals who have participated on pharmaceutical companies’ Speakers Bureaus should be prohibited from DSM panel membership, and 2) There should be a rebuttable presumption of prohibiting COIs among the DSM work groups and task force. If individuals with the requisite expertise are not available, individuals with associations to industry could consult to the DSM panels, but they would not have decision-making authority on revisions or inclusion of new disorders (7).
As our present findings are concerning, these recommendations are even more pressing today. As researchers, clinicians, policymakers, and leaders in evidence-based medicine have argued (17), guideline writers should be free of financial relationships with industry, especially those writers who are responsible for the preeminent psychiatric diagnostic manual in the world.
MedicalResearch.com: Is there anything else you would like to add?
Response: The findings here are likely an under-estimate of the renumeration received because 45.2% of working group members include PhDs and others who were are not covered by Open Payments (11).
Reputable journals (e.g. JAMA, NEJM) and even some textbooks (18) are transparent about COI. We are cautiously optimistic that a future study about the DSM-6 will be unnecessary as the APA will voluntarily collect, verify, and disclose this information (11).
This research received no external funding. A middle-author (BJP) was part of osteoarthritis research team supported by Pfizer and Eli Lilly (2019-2021). All other authors have no competing interests.
The raw data is publicly available at: https://openpaymentsdata.cms.gov/ (11). We recommend that anyone that is concerned about COIs by their healthcare providers make frequent use of this very accessible database.
References
- Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines; Graham R, Mancher M, Miller Wolman D, et al., editors. Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press (US);Available from: https://www.ncbi.nlm.nih.gov/books/NBK209539/?report=classic doi: 10.17226/13058
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift?. JAMA. 2000;283(3):373-380. doi:10.1001/jama.283.3.373
- Moore DA, Tanlu L, Bazerman MH. Conflict of interest and the intrusion of bias. Judgment and Decision Making 2010;5:37–53. doi:10.1017/s1930297500002023.
- Dana J. A social science perspective on gifts to physicians from industry. JAMA 2003;290:252. doi:10.1001/jama.290.2.252.
- Nejstgaard CH, Bero L, Hróbjartsson A, et al. Association between conflicts of interest and favourable recommendations in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews: systematic review. BMJ. 2020;371:m4234. Published 2020 Dec 9. doi:10.1136/bmj.m4234
- Thompson DF. The challenge of conflict of interest in medicine. Zeitschrift Für Evidenz, Fortbildung Und Qualität Im Gesundheitswesen 2009;103:136–40. doi:10.1016/j.zefq.2009.02.021.
- Cosgrove L, Krimsky S, Vijayaraghavan M, Schneider L. Financial ties between DSM-IV panel members and the pharmaceutical industry. Psychother Psychosom. 2006;75(3):154-160. doi:10.1159/000091772
- Cosgrove L, Krimsky S. A comparison of DSM-IV and DSM-5 panel members’ financial associations with industry: a pernicious problem persists. PLoS Med. 2012;9(3):e1001190. doi:10.1371/journal.pmed.1001190
- Davis LC, Diiani AT, Drumheller SR, Elansary NN, D’Ambrozio GN, Herrawi F, et al. A cross-sectional analysis of undisclosed financial conflicts of interest in DSM-5-TR: Caveat emptor. British Medical Journal. 2024; https://www.bmj.com/content/384/bmj-2023-076902.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text rev. 2022.
- Centers for Medicare & Medicaid services [Internet]. Baltimore, MD (USA): Open Payments Newly Added Covered Recipients; 2023 [updated 2023; cited 2023 Mar 6]. Available from: Centers for Medicare & Medicaid services [Internet]. Baltimore, MD (USA): Open Payments Program participants; 2023 [updated 2023; cited 2023 Mar 6]. Available from: https://www.cms.gov/OpenPayments/Program-Participants
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289(4):454-465. doi:10.1001/jama.289.4.454
- Stelfox HT, Chua G, O’Rourke K, Detsky AS. Conflict of interest in the debate over calcium-channel antagonists. N Engl J Med. 1998;338(2):101-106. doi:10.1056/NEJM199801083380206
- Krimsky S. Combating the funding effect in science: What’s beyond transparency? Stanford Law Policy Rev. 2010;XXI:101–123.
- Cosgrove L, Bursztajn HJ, Erlich DR, Wheeler EE, Shaughnessy AF. Conflicts of interest and the quality of recommendations in clinical guidelines. J Eval Clin Pract. 2013;19(4):674-681. doi:10.1111/jep.12016
- Lexchin J, O’Donovan O. Prohibiting or ‘managing’ conflict of interest? A review of policies and procedures in three European drug regulation agencies. Soc Sci Med. 2010;70(5):643-647. doi:10.1016/j.socscimed.2009.09.002
- Grande D, Frosch DL, Perkins AW, Kahn BE. Effect of exposure to small pharmaceutical promotional items on treatment preferences. Arch Intern Med. 2009;169(9):887-893. doi:10.1001/archinternmed.2009.64
- Stahl SM, Grady MM, Muntner N. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 5th Cambridge: Cambridge University Press; 2021.
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition.
Some links may be sponsored. Products are not endorsed.
Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on January 14, 2024 by Marie Benz MD FAAD
