06 Apr Teprotumumab-Tepezza Reduces Proptosis in Patients with Thyroid Eye Disease
MedicalResearch.com Interview with:
Dr. Raymond Douglas MD PhD
Board Certified Oculoplastic Surgeon
Beverly Hills, CA
MedicalResearch.com: What is the background for this study? Would you briefly explain what is meant by proptosis? How does teprotumumab work?
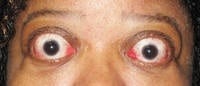
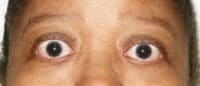
Response: This study provides pooled efficacy data from the Phase 2 and 3 clinical trials of teprotumumab showing that the recently FDA-approved medicine effectively reduces proptosis, also known as eye bulging, in patients with Thyroid Eye Disease (TED) regardless of age, gender and smoking status. Proptosis is one of the most debilitating symptoms of TED, especially given the accompanying pain, vision impairment and emotional distress.
Teprotumumab is a fully human monoclonal antibody and a targeted inhibitor of the IGF-1 receptor. In patients with Thyroid Eye Disease, the IGF-1 receptor is overexpressed on orbital tissues and when activated, causes inflammation and enlargement of ocular muscles, expansion of orbital tissue and fat and forward displacement of the eye, resulting in eye bulging. The proteins in teprotumumab target and bind to the IGF-1 receptor and inhibit its function, thereby reducing inflammation, preventing tissue expansion behind the eye, and preventing muscle and fat tissue remodeling. Based on this mechanism of action, it is believed that teprotumumab addresses the underlying biology of the disease.
MedicalResearch.com: What are the main findings?
Response: The data show that teprotumumab effectively reduces proptosis in patients with Thyroid Eye Disease regardless of age, gender and smoking status. Specifically, at week 24 of treatment across all subgroups, significantly more patients receiving teprotumumab experienced a clinically meaningful improvement of at least 2 mm in proptosis compared to those receiving placebo.
Of note, we know that smoking is a significant risk-factor for TED and can make it more serious and difficult to manage. In this analysis, we found that 70 percent of smokers in the teprotumumab treatment group were proptosis responders, with a mean reduction of almost 3 millimeters – which is comparable to results seen in non-smokers.
MedicalResearch.com: What should readers take away from your report?
Response: This analysis provides further evidence for physicians to consider when determining the type of patient to treat with teprotumumab. By understanding that teprotumumab effectively reduces proptosis in patients with thyroid eye disease regardless of age, gender and smoking status, physicians can make even more informed decisions when treating their patients.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: There is a current ongoing open-label extension study of the teprotumumab Phase 3 clinical trial called OPTIC-X. The OPTIC-X trial is designed to better understand whether certain patients may benefit from retreatment or longer treatment (more than six months) with teprotumumab. OPTIC-X is a 48-week, open-label extension study in which patients who participated in the OPTIC Phase 3 clinical trial may receive up to eight additional infusions of teprotumumab. The primary endpoint is proptosis responder rate (the percentage of participants with ≥2 mm reduction in proptosis in the study eye) without deterioration (≥2 mm increase) of proptosis in the fellow eye.
MedicalResearch.com: Is there anything else you would like to add?
Response: Teprotumumab, commercially known as TEPEZZA, is the first and only FDA-approved medicine for TED, fulfilling a serious unmet need for patients living with this debilitating disease who historically have had to suffer in pain as their symptoms progress – risking severe and permanent damage to their eyes. Teprotumumab is the only medicine that has been shown to address the underlying biology of the disease, and significantly improve eye bulging and double vision.
I am an active consultant for Horizon and Immunovant.
Citations:
ENDO2020 abstract and
George Kahaly, MD, PhD, Raymond Douglas, MD, PhD, Robert Holt, PharmD, MBA, Megan Francis-Sedlak, PhD, Renee Perdok, PhD, Saba Sile, MD, Terry Smith, MD, SAT-554 Teprotumumab in Graves’ Orbitopathy: Extended Outcome Analyses, Journal of the Endocrine Society, Volume 3, Issue Supplement_1, April-May 2019, SAT–554, https://doi.org/10.1210/js.2019-SAT-554
JOIN OUR EMAIL LIST
[mailpoet_form id="5"]We respect your privacy and will never share your details.
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on April 6, 2020 by Marie Benz MD FAAD