13 May Trichomoniasis: Results of Phase 3 Trial to Assess Efficacy and Safety of Single-Dose Solosec® (secnidazole) in Women
MedicalResearch.com Interview with:

Dr. Kaufman
Gregory Kaufman, M.D.
Senior Vice President
Global Clinical and Medical Affairs
Specialty at Lupin
MedicalResearch.com: What is the background for this study? Would you briefly explain what is meant by trichomoniasis? How common is this infection?
Response: The Phase 3 trial evaluated the effectiveness and safety of a single oral dose of Solosec® (secnidazole) 2g oral granules for the treatment of trichomoniasis in adult women. Top-line results were positive and showed that Solosec was generally well-tolerated.
Trichomoniasis is the most common non-viral sexually transmitted infection (STI) in the U.S., and is caused by a protozoan parasite called Trichomonas vaginalis.[i] Trichomoniasis affects 3 to 5 million people in the U.S.,[ii] and is four- to five-times more prevalent in in women, compared to men.[iii].

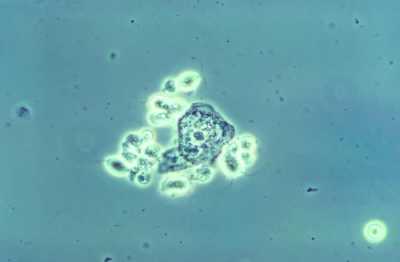
This photomicrograph of a wet mount-prepared vaginal discharge specimen, revealed the presence of a grouping of Trichomonas vaginalis parasitic protozoa, in this case of trichomoniasis. CDC Image
MedicalResearch.com: What are the main findings?
- Lupin announced positive top-line results from its pivotal Phase 3 clinical trial to assess efficacy and safety of single-dose Solosec® (secnidazole) 2g oral granules in 147 female patients with trichomoniasis, a common sexually transmitted infection (STI)
- The trial met its primary endpoint of microbiological cure at the test-of-cure (TOC) visit on study Day 6-12, defined as a negative Trichomonas vaginalis (T. vaginalis) culture
- The predefined primary efficacy endpoint, defined as Microbiological Cure (i.e., InPouch™ TV test negative for T. vaginalis) at the TOC visit (Day 6-12) in the modified Intent-To-Treat (mITT) population (all randomized subjects who were culture positive for T. vaginalis and negative for gonorrhea and chlamydia at baseline), was 92.2% (59/64) for Solosec versus 1.5% (1/67) for placebo (p<0.001)
- In the Per-Protocol population, the cure rate was 94.9% (56/59) for Solosec versus 1.7% (1/60) for placebo (p<0.001)
- Solosec was generally well-tolerated with the most commonly reported adverse events being vulvovaginal candidiasis (2.7%) and nausea (2.7%). No serious adverse events were observed.
- The American College of Gynecology (ACOG) currently recommends a twice-daily for seven day treatment or alternatively a single-dose treatment.ii However, trials have shown 7-day regimens to be more effective, with higher cure rates.[iv] In addition, in real-world settings, treatment failures and reinfection rates are high, and medication non-compliance is a common cause.iv
MedicalResearch.com: How does Solosec® differ from other treatments for this disorder?
Response: Solosec is currently approved by the U.S. FDA to treat bacterial vaginosis in adult women.[v] It has not been evaluated by the FDA for the treatment of Trichomoniasis. The company plans to submit a supplemental New Drug Application (sNDA) to the FDA in 2H2020 for review.
MedicalResearch.com: What should readers take away from your report?
Response: We are encouraged by the top-line findings from the Phase 3 study and plan to submit the full results for presentation at an upcoming medical meeting. Based on the full analysis, we plan to submit for evaluation, a supplemental New Drug Application to the FDA in the second half of this year for Solosec to treat trichomoniasis with a single dose.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Additional information on the disparities regarding the increased risk of contracting trichomoniasis among certain populations would further help us understand the disease. For example, African American women have a nearly ten times higher risk of being affected compared with non-Hispanic white women.iii Trichomoniasis is four- to five-times more prevalent in women compared to men.iii In addition, trichomoniasis can increase the risk of contracting or spreading other sexually transmitted infections and it is associated with a two- to three-fold increased risk of HIV infection. [vi],[vii] Trichomoniasis is also associated with adverse reproductive health outcomes, including infertility and preterm birth.iv
MedicalResearch.com: Is there anything else you would like to add?
Response: Lupin is committed to developing treatments for conditions like trichomoniasis, which impact millions of women and we are encouraged by the top-line findings from the Phase 3 study.
References:
[i] Centers for Disease Control and Prevention. Trichomoniasis – CDC Fact Sheet. Available at: https://www.cdc.gov/std/trichomonas/stdfact-trichomoniasis.htm. Accessed April 21, 2020.
[ii] American College of Obstetricians and Gynecologists. Vaginitis in Nonpregnant Patients. ACOG Practice Bulletin No. 215. Obstet Gynecol 2020;135(1):e1-17.
[iii] Flagg EW, Meites E, Phillips C, Papp J, Torrone EA. Prevalence of Trichomonas vaginalis Among Civilian, Noninstitutionalized Male and Female Population Aged 14 to 59 Years: United States, 2013 to 2016. Sex Transm Dis. 2019;46(10): e93–e96. doi:10.1097/OLQ.0000000000001013.
[iv] Sobel JD, Mitchell C. Trichomoniasis. UpToDate. Available at: https://www.uptodate.com/contents/trichomoniasis. Accessed April 22, 2020.
[v] SOLOSEC [prescribing information]. Baltimore, MD: Lupin Pharmaceuticals, Inc; 2019.
[vi] McClelland, RS. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. Journal of Infectious Diseases. 2007 Mar 1;195(5):698-702.
[vii] Van Der Pol, B. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. Journal of Infectious Diseases. 2008 Feb;197(4):548-54.
JOIN OUR EMAIL LIST
[mailpoet_form id="5"]We respect your privacy and will never share your details.
[last-modified]
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, endorse or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website
Last Updated on May 13, 2020 by Marie Benz MD FAAD
