Author Interviews, Cancer Research, Prostate Cancer / 03.06.2019
SPARTAN Study: Older Castration-Resistant Prostate Cancer Patients Can Tolerate and Benefit from Apalutamide (ERLEADA®)
MedicalResearch.com Interview with:
Julie N. Graff, MD
Associate Professor of Medicine
Knight Cancer Institute
Chief of Hematology/Oncology
VA Portland Health Care System
MedicalResearch.com: What is the background for this study?
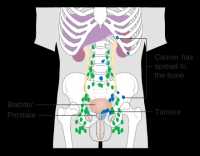
Response: Androgen deprivation therapy is often deployed in patients with a rising PSA after local therapy (such as radical prostatectomy or radiation therapy). With time, the prostate cancer can develop resistance to ADT, at which point it is called castration resistant prostate cancer (CRPC). There were 6 treatments for metastatic CRPC that have shown improved survival. However, in non-metastatic disease, there was nothing that showed improved survival.
The SPARTAN study was designed to determine if a next generation androgen receptor antagonist could delay the time to metastatic disease. Overall survival was a secondary endpoint. (more…)