Author Interviews, Brigham & Women's - Harvard, JAMA, Prostate Cancer, Radiation Therapy, Surgical Research / 16.11.2018
Advanced Prostate Cancer: Risk of Mortality with Surgery vs Radiotherapy
MedicalResearch.com Interview with:
Anthony Victor D'Amico, MD, PhD
Professor and Chief,
Genitourinary Radiation Oncology
Harvard Medical School
MedicalResearch.com: What is the background for this study?
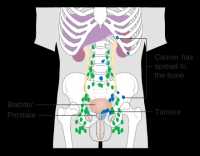
Response: This study investigated whether surgery followed by the use of adjuvant low dose radiation and short course hormonal therapy as compared to high dose radiation and hormonal therapy could provide an equivalent low risk of death from prostate cancer amongst men presenting with aggressive and not infrequently fatal Gleason score 9 or 10 prostate cancer.
It has been shown previously (https://jamanetwork.com/journals/jama/fullarticle/2673969) and validated in the current study that surgery alone in such cases leads to a more then 2.5-fold increase in the risk of death from prostate cancer as compared to high dose radiation and hormonal therapy. (more…)