MedicalResearch.com Interview with:
Hsien-Yuan Lane, MD,PhD
Distinguished Professor, Director, Graduate Institute of Biomedical Sciences
China Medical University, Taichung, Taiwan
Director, Brain Diseases Research Center (BDRC), Addiction Research Center, and Department of Psychiatry,
China Medical University and Hospital, Taichung, Taiwan
PI, Taiwan Clinical Trial Consortium for Mental Disorders
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Schizophrenia is a chronic and severe mental illness affecting more than 21 million people worldwide. Clozapine has been regarded as the last-line antipsychotic agent for patients with refractory schizophrenia. However, an estimated 40–70% of patients with refractory schizophrenia fail to improve even with clozapine , referred to as “clozapine-resistant”. To date, there is no convincing evidence for augmentation on clozapine.
Activation of N-methyl-D-aspartate (NMDA) receptors, including inhibition of D-amino acid oxidase (DAAO) that may metabolize D-amino acids, has been reported to be beneficial for patients receiving antipsychotics other than clozapine.
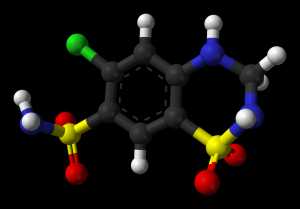
Sodium benzoate is a DAAO inhibitor. A recent randomized, double-blind, placebo-controlled clinical trial found that add-on sodium benzoate improved the clinical symptoms in patients with clozapine-resistant schizophrenia, possibly through DAAO inhibition and antioxidation as well.
(more…)