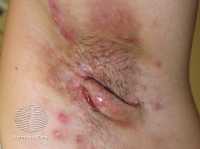
Editors' note: Be sure to follow up with recommended colonoscopy examinations. If you are having bowel or rectal symptoms, consult your health care provider before relying on home remedies.
 When you're in the grip of hemorrhoid pain, relief can't come soon enough. It’s a common misconception that hemorrhoids are untreatable. However, there are numerous strategies you can employ to prevent and manage hemorrhoids, offering a glimmer of hope and empowerment in your journey to wellness.
Hemorrhoids, depending on their position, can cause cases of bleeding, itching, soreness, or pain. Internal hemorrhoids lead to painless bleeding, while external hemorrhoids lead to pain. Below are the five tips that can assist in reducing hemorrhoid discomfort.
When you're in the grip of hemorrhoid pain, relief can't come soon enough. It’s a common misconception that hemorrhoids are untreatable. However, there are numerous strategies you can employ to prevent and manage hemorrhoids, offering a glimmer of hope and empowerment in your journey to wellness.
Hemorrhoids, depending on their position, can cause cases of bleeding, itching, soreness, or pain. Internal hemorrhoids lead to painless bleeding, while external hemorrhoids lead to pain. Below are the five tips that can assist in reducing hemorrhoid discomfort.
 When you're in the grip of hemorrhoid pain, relief can't come soon enough. It’s a common misconception that hemorrhoids are untreatable. However, there are numerous strategies you can employ to prevent and manage hemorrhoids, offering a glimmer of hope and empowerment in your journey to wellness.
Hemorrhoids, depending on their position, can cause cases of bleeding, itching, soreness, or pain. Internal hemorrhoids lead to painless bleeding, while external hemorrhoids lead to pain. Below are the five tips that can assist in reducing hemorrhoid discomfort.
When you're in the grip of hemorrhoid pain, relief can't come soon enough. It’s a common misconception that hemorrhoids are untreatable. However, there are numerous strategies you can employ to prevent and manage hemorrhoids, offering a glimmer of hope and empowerment in your journey to wellness.
Hemorrhoids, depending on their position, can cause cases of bleeding, itching, soreness, or pain. Internal hemorrhoids lead to painless bleeding, while external hemorrhoids lead to pain. Below are the five tips that can assist in reducing hemorrhoid discomfort.