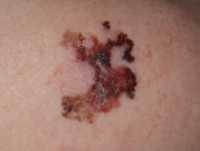
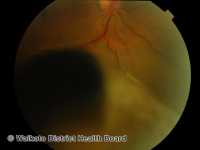
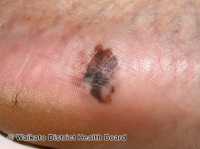
Melanoma: Gene Profile Test Can Help Pathologists Identify Difficult-To-Diagnose Lesions
Targeted Combination Immunotherapy Improved Survival in Stage III Melanoma
Adjuvant Therapy in Stage IIIA Melanoma: Cost vs Benefit of Gene Profiling
Melanoma: Florescent Biomarkers Detectable in Urine
Melanoma: Bioresorbable Skin Patch Developed to Deliver Prolonged Local Chemotherapy
Genes Linked to Melanoma of the Eye Identified
Racial and Ethnic Disparities in Melanoma Awareness
New Gene Regions Linked to Melanoma Risk Identified
AI Improved Diagnosis of Skin Disorders, especially Distinguishing Benign from Malignant Tumors
New Treatments Linked to Drop in Mortality from Malignant Melanoma
Stress and Delayed Detection Contribute to Increased Melanoma Mortality with Loss of Partner
Which States Have Most Ultraviolet-Related Melanomas?
Melanoma Cells Change Their Cytoskeleton to Evade Treatment
Sun Protection Rising? Melanoma Incidence Decreasing in Young Adults
Blood Biomarker Can Help Distinguish Malignant from Benign Ocular Moles
Bariatric Surgery Linked to Reduced Melanoma Risk
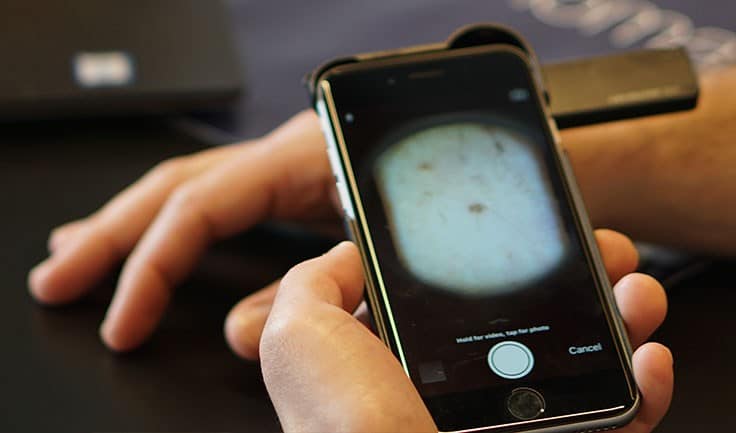
DERM: AI Algorithm Helps Determine Likelihood of Melanoma from Dermoscopic Images
 Dr. Helen Marsden PhD
Skin Analytics Limited
London, United Kingdom
MedicalResearch.com: What is the background for this study?
Response: In this technology age, with the explosion of interest and applications using Artificial Intelligence, it is easy to accept the output of a technology-based test - such as a smartphone app designed to identify skin cancer - without thinking too much about it. In reality, technology is only as good as the way it has been developed, tested and validated. In particular, AI algorithms are prone to a lack of “generalisation” - i.e. their performance drops when presented with data it has not seen before. In the medical field, and particularly in areas where AI is being developed to direct a patient’s diagnosis or care, this is particularly problematic. Inappropriate diagnosis or advice to patients can lead to false reassurance, heightened concern and pressure on NHS services, or worse. It is concerning, therefore, that there are a large number of smartphone apps available that provide an assessment of skin lesions, including some that provide an estimate of the probability of malignancy, that have not been assessed for diagnostic accuracy.
Skin Analytics has developed an AI-based algorithm, named: Deep Ensemble for Recognition of Malignancy (DERM), for use as a decision support tool for healthcare providers. DERM determines the likelihood of skin cancer from dermoscopic images of skin lesions. It was developed using deep learning techniques that identify and assess features of these lesions which are associated with melanoma, using over 7,000 archived dermoscopic images. Using these images, it was shown to identify melanoma with similar accuracy to specialist physicians. However, to prove the algorithm could be used in a real life clinical setting, Skin Analytics set out to conduct a clinical validation study.
(more…)
Dr. Helen Marsden PhD
Skin Analytics Limited
London, United Kingdom
MedicalResearch.com: What is the background for this study?
Response: In this technology age, with the explosion of interest and applications using Artificial Intelligence, it is easy to accept the output of a technology-based test - such as a smartphone app designed to identify skin cancer - without thinking too much about it. In reality, technology is only as good as the way it has been developed, tested and validated. In particular, AI algorithms are prone to a lack of “generalisation” - i.e. their performance drops when presented with data it has not seen before. In the medical field, and particularly in areas where AI is being developed to direct a patient’s diagnosis or care, this is particularly problematic. Inappropriate diagnosis or advice to patients can lead to false reassurance, heightened concern and pressure on NHS services, or worse. It is concerning, therefore, that there are a large number of smartphone apps available that provide an assessment of skin lesions, including some that provide an estimate of the probability of malignancy, that have not been assessed for diagnostic accuracy.
Skin Analytics has developed an AI-based algorithm, named: Deep Ensemble for Recognition of Malignancy (DERM), for use as a decision support tool for healthcare providers. DERM determines the likelihood of skin cancer from dermoscopic images of skin lesions. It was developed using deep learning techniques that identify and assess features of these lesions which are associated with melanoma, using over 7,000 archived dermoscopic images. Using these images, it was shown to identify melanoma with similar accuracy to specialist physicians. However, to prove the algorithm could be used in a real life clinical setting, Skin Analytics set out to conduct a clinical validation study.
(more…)Cancer Cells Take Advantage of Brain’s Fatty Environment to Grow
Australia’s SunSmart Program Program Linked to Decrease in Melanomas
Indoor Tanning More Available in Neighborhoods with More Gay Men
Genetics Plays A Key Role in Where Moles Develop on Skin
Nanovaccine Opens New Approach to Melanoma Treatment
Circulating DNA Can Indicate Melanoma Treatment Failure
How Much of Your Sunscreen is Absorbed?
Pediatric Melanoma Risk Increasing in Adolescents & Young Adults, Including in Non-Whites
Sentinel Lymph Node Drainage Can Be Used to Test For Marker of Melanoma Relapse Risk
Melanoma Rates Stable in Australia but Rising in US
Dermatologist Discusses Personalized Approach to Skin Cancer Treatment
MedicalResearch.comInterview with:

Jac Dinnes PhD, MSc, MA, PGDip
Senior Researcher
Test Evaluation Research Group
Institute of Applied Health Research
University of Birmingham
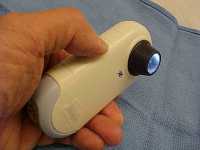
MedicalResearch.com: What is thebackground for this study? Would you briefly explain the benefits of dermoscopy?
Response: This systematic review was one of a series of Cochrane Reviews of studies evaluating different tests for diagnosing skin cancer. Within creasing rates of skin cancer and an increasing number of more specialised tests becoming available in both primary care and in specialist settings, a thorough review of all available evidence was timely.
The diagnosis of melanoma and other skin cancers fundamentally relies on clinical examination, including history taking, and visual inspection of the concerning skin lesion (mole or patch of skin) and surrounding skin (and other lesions). A dermatoscope is a handheld device using visible light (such as from incandescent or LED bulbs), that allows more detailed examination of the skin compared to examination by the naked eye alone.
Knowing the diagnostic accuracy of dermoscopy added to visual inspection alone, across a range of observers and settings, is critical to understanding its contribution for the diagnosis of melanoma and to future understanding of the potential role of the growing number of other high-resolution image analysis techniques.
(more…)