MedicalResearch.com Interview with:
Dr. Douglas Maslin, MPhil, MB BCHir
Dermatologist and Pharmacologist
Addenbrooke's Hospital
Cambridge, UK
MedicalResearch.com: What is the background for this study?
Response: I’d like to answer this question in three parts:
 Firstly
Firstly, the background to Evelo and the therapeutic EDP1815: Evelo is developing orally administered biologic medicines based on a new understanding of how systemic inflammation is controlled. Evelo’s medicines are selected for their ability to modulate the small intestinal axis, or SINTAX, a network of anatomical and functional connections that has evolved to connect the small intestine with the rest of the body. SINTAX links small intestinal mucosal immunology with systemic inflammation and is now accessible with oral medicines. This inflammatory control pathway may enable a new class of products which are effective, safe, and can be manufactured affordably at large scale.
EDP1815 is a non-live pharmaceutical preparation of a strain of the bacterium
Prevotella histicola isolated from the duodenum of a human donor
. Its pharmacodynamic effect is through interactions with the immune cells within the small intestine and it has no systemic absorption. These local interactions in the small intestine then downregulate systemic inflammation. In fact, the inflammatory control afforded by targeting the small intestinal axis appears to result in the coordinated downregulation of multiple inflammatory pathways without immunosuppression, mimicking the body’s normal physiological processes of inflammation resolution.
Secondly, there is the key and exciting background pre-clinical data on EDP1815 – the details of which have been published today at the EADV conference. For example, oral administration of EDP1815 to mice has been shown to lead to striking therapeutic effects in
in vivo models of delayed-type hypersensitivity, imiquimod-induced skin inflammation, fluorescein isothiocyanate cutaneous hypersensitivity, collagen-induced arthritis, and experimental acute encephalomyelitis (EAE).
The consistency of effect and dose shows that EDP1815 can coordinately resolve systemic inflammation across TH1, TH2 and TH17 pathways. This suggests the potential for clinical benefit across multiple diseases.
And,
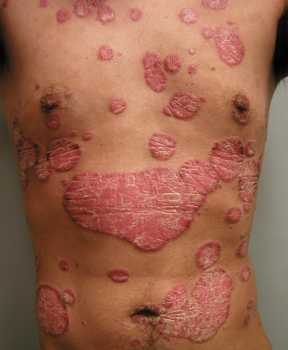
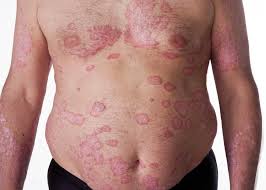
thirdly, there is the clinical unmet need for an oral, safe, effective treatment specifically for mild and moderate psoriasis patients, who have very limited treatment options outside of the poorly tolerated topical therapies, and these patients are reported to be dissatisfied with treatment options and therefore are often under-treated.
These three points explain the background to EDP1815 and the reason for progressing forward into the phase 1b in psoriasis.
(more…)