Pharmaceutical Companies, Pharmacology / 14.02.2025
How Antibody Drug Discovery is Transforming Rare Disease Research
Although each rare disease is uncommon on its own, together, they represent a major global health challenge. With over 7,000 known rare diseases, the global impact is substantial. Currently, around 300 million people worldwide are living with a rare disease.
Unlike more prevalent conditions, rare diseases often receive less research funding, making drug development a lengthy and challenging process. However, recent advancements in antibody drug discovery are transforming this landscape, offering new hope to millions of patients.
Antibody drug discovery is an advanced biotechnology field that utilizes the immune system to develop precise treatments. Scientists identify and isolate specific antibodies capable of binding to disease-causing targets. This approach enables the creation of drugs that directly address the root cause of rare diseases, ensuring more effective and personalized therapies.
In this article, we will explore the transformative impact of antibody drug discovery on rare disease research.










 Lauren C. Davis, MBS
Department of Medical Education
Geisinger Commonwealth School of Medicine
Scranton, PA 19409
MedicalResearch.com: What is the background for this study?
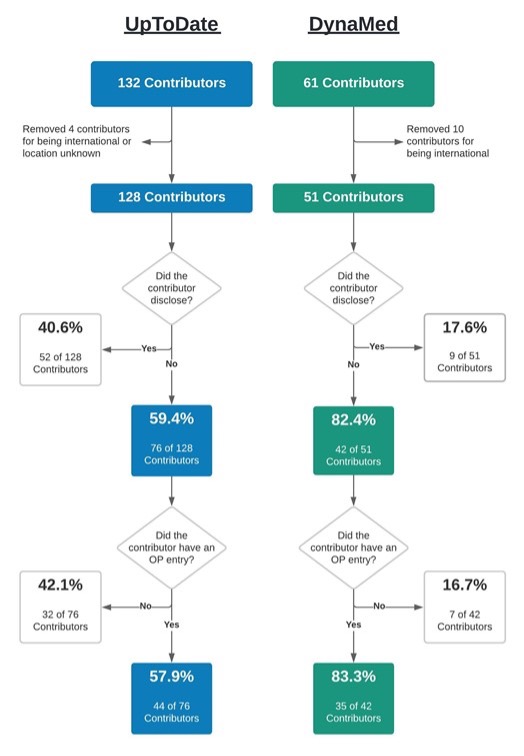
Response: Financial conflicts of interest (COIs) resulting from ties between academia and industry have been under scrutiny for their potential to hinder the integrity of medical research. COIs can lead to implicit bias, compromise the research process, and erode public trust (1-6). The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM), standardizes symptom criteria and codifies psychiatric disorders. This manual contributes to the approval of new drugs, extensions of patent exclusivity, and can influence payers and mental health professionals seeking third-party reimbursements. Given the implications of the DSM on public health, it is paramount that it is free of industry influence. Previous research has shown a high prevalence of industry ties among panel and task force members of the DSM-IV-TR and DSM-5, despite the implementation of a disclosure policy for the DSM-5 (7,8). This study (9) determined the extent and type of COIs received by panel and task-force members of the DSM-5-TR (2022) (10). As the DSM-5-TR did not disclose COI, we used the Center for Medicare and Medicaid Services Open Payments (OP) database (11) to quantify them.
Lauren C. Davis, MBS
Department of Medical Education
Geisinger Commonwealth School of Medicine
Scranton, PA 19409
MedicalResearch.com: What is the background for this study?
Response: Financial conflicts of interest (COIs) resulting from ties between academia and industry have been under scrutiny for their potential to hinder the integrity of medical research. COIs can lead to implicit bias, compromise the research process, and erode public trust (1-6). The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM), standardizes symptom criteria and codifies psychiatric disorders. This manual contributes to the approval of new drugs, extensions of patent exclusivity, and can influence payers and mental health professionals seeking third-party reimbursements. Given the implications of the DSM on public health, it is paramount that it is free of industry influence. Previous research has shown a high prevalence of industry ties among panel and task force members of the DSM-IV-TR and DSM-5, despite the implementation of a disclosure policy for the DSM-5 (7,8). This study (9) determined the extent and type of COIs received by panel and task-force members of the DSM-5-TR (2022) (10). As the DSM-5-TR did not disclose COI, we used the Center for Medicare and Medicaid Services Open Payments (OP) database (11) to quantify them.