29 Jan Gene Therapy Restores Hearing In Trial of Autosomal Recessive Pediatric Deafness
Posted at 00:00h
in Author Interviews, Brigham & Women's - Harvard, Genetic Research, Hearing Loss, Lancet, Pediatrics
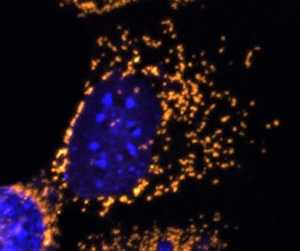
There has never been any treatment for any type hearing loss, including genetic hearing loss. This is the first time that a child born with complete hearing loss can regain hearing after gene therapy.