Author Interviews, Columbia, Genetic Research, Hematology, NEJM / 16.12.2021
Gene Additive Therapy Prevents Pain Crisis Episodes in Sickle Cell Patients
MedicalResearch.com Interview with:
Markus Y Mapara, MD
Professor of Medicine
Director of the Blood and Marrow Transplantation
Columbia University Medical Center
MedicalResearch.com: What is the background for this study? What are the main findings?
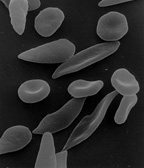
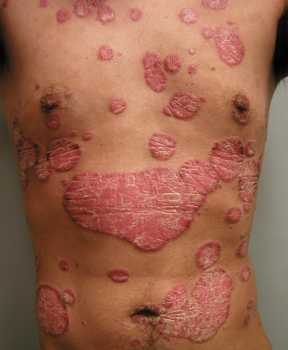
Response: Sickle cell disease is caused by a point mutation in the beta-globin gene of hemoglobin resulting in the production of abnormal hemoglobin which leads to formation of sickle-shaped RBC under conditions of low oxygen. Sickle cell disease affects about 100,000 patients in the US which are predominantly African American. The only curative approach is to perform an allogeneic bone marrow transplant which is however fraught with significant treatment-related risks if a matched sibling donor is not available.
The current study describes the successful application of a novel gene therapy to treat patients with sickle cell disease. The strategy is based on a gene-addition approach to introduce the genetic information for a Hemoglobin F-like molecule termed HgAT87Q into hematopoietic stem cells. The expression of this novel hemoglobin prevents polymerization of HgbS and has now been demonstrated to prevent the occurrence of vaso-occlusive pain crises in sickle cell disease patients.
(more…)