Author Interviews, Cancer Research, CDC, JAMA, Lung Cancer / 10.12.2020
More Lung Cancer in Women Non-Smokers Than Men, But Most Lung Cancer Still in Smokers
MedicalResearch.com Interview with:
David A. Siegel, MD, MPH
Division of Cancer Prevention and Control
US Centers for Disease Control and Prevention
Atlanta, Georgia
MedicalResearch.com: Why is it important to better understand the smoking histories (both current/former and never smokers) among lung cancer patients?
Response: Knowledge of smoking status of patients diagnosed with lung cancer can help us understand how to best prevent, detect, and treat lung cancer in the future.
More than 84% of women and 90% of men newly diagnosed with lung cancer had ever smoked cigarettes, and half of patients aged 20 to 64 years newly diagnosed with lung cancer were current cigarette smokers. These findings reinforce the importance of cigarette cessation and lung cancer screening.
1 out of every 8 people diagnosed with lung cancer had never smoked cigarettes, which reiterates the importance of learning more about their risk factors for lung cancer, which could impact prevention and treatment. (more…)





 Response: LENVIMA (lenvatinib), discovered and developed by Eisai, is an orally available multiple receptor tyrosine kinase inhibitor that inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). LENVIMA inhibits other kinases that have been implicated in pathogenic angiogenesis, tumor growth, and cancer progression in addition to their normal cellular functions, including fibroblast growth factor (FGF) receptors FGFR1-4, the platelet derived growth factor receptor alpha (PDGFRα), KIT, and RET.
LENVIMA is approved in combination with everolimus for the treatment of patients with advanced renal cell carcinoma (RCC) following one prior anti-angiogenic therapy. The approved starting dose for LENVIMA is 18 mg daily. The objective of Study 218, a randomized, open-label, Phase 2 trial, was to assess whether the lower starting dose of LENVIMA (14 mg daily) in combination with everolimus (5 mg daily) would provide similar efficacy with an improved safety profile compared to the FDA-approved starting dose of LENVIMA (18 mg daily) plus everolimus (5 mg daily) in patients with advanced renal cell carcinoma (RCC) following prior treatment with an antiangiogenic therapy.
In the US, LENVIMA is also indicated for:
Response: LENVIMA (lenvatinib), discovered and developed by Eisai, is an orally available multiple receptor tyrosine kinase inhibitor that inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). LENVIMA inhibits other kinases that have been implicated in pathogenic angiogenesis, tumor growth, and cancer progression in addition to their normal cellular functions, including fibroblast growth factor (FGF) receptors FGFR1-4, the platelet derived growth factor receptor alpha (PDGFRα), KIT, and RET.
LENVIMA is approved in combination with everolimus for the treatment of patients with advanced renal cell carcinoma (RCC) following one prior anti-angiogenic therapy. The approved starting dose for LENVIMA is 18 mg daily. The objective of Study 218, a randomized, open-label, Phase 2 trial, was to assess whether the lower starting dose of LENVIMA (14 mg daily) in combination with everolimus (5 mg daily) would provide similar efficacy with an improved safety profile compared to the FDA-approved starting dose of LENVIMA (18 mg daily) plus everolimus (5 mg daily) in patients with advanced renal cell carcinoma (RCC) following prior treatment with an antiangiogenic therapy.
In the US, LENVIMA is also indicated for:





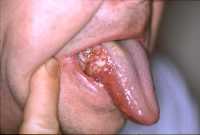



![MedicalResearch.com Interview with: Selin Tokez, PhD Student Department of Dermatology Erasmus MC, Rotterdam MedicalResearch.com: What is the background for this study? Response: Cutaneous squamous cell carcinoma (cSCC) is the second most common skin cancer worldwide with still increasing incidence rates. Given these high incidence rates together with the associated health costs and possibility of fatal progression, it is extremely important to have accurate and complete data on the epidemiology of this disease. Nevertheless, national cancer registries in many countries do not routinely record cSCC cases and therefore currently known numbers are mainly based on incomplete data sources. Additionally, if cSCC cases are registered, this usually only concerns the first cSCC per patient while we know that, contrary to many other malignant neoplasms, patients may develop numerous cSCCs over time. MedicalResearch.com: What are the main findings? Response: In the present study, we analyzed Dutch nationwide data comprising about 145,000 patients with a first invasive cSCC diagnosis between the years 1989 and 2017. We found that the incidence rates of a first cSCC per patient almost tripled in male patients and increased about fivefold in female patients in this 30-year time period. Also, we had data on all cSCCs per patient for the year 2017 and could therefore compare this with the data on only the first cSCC per patient: incidence rates increased by 58% for men and 35% for women when multiple cSCCs were considered. In absolute numbers, this resulted in an increase of 45% in cSCC diagnoses in 2017. Lastly, we extended our analyses by predicting future cSCC incidence rates up to 2027. Given that no substantially effective measures are undertaken in the near future, current cSCC incidence rates will increase with 23% in males and 29% in females in the next decade. MedicalResearch.com: What should readers take away from your report? Response: We could summarize our main message in two points: while people generally know that cSCC is a very common disease with increasing incidence rates, it is not taken into account that these numbers are often based on incomplete data registries and that the real numbers are even higher. In this paper, we provided these numbers for a period of 30 years based on highly accurate data from the Netherlands Cancer Registry. On top of that, as a second main finding, we showed that the real burden caused by cSCC is approximately 50% higher (taken males and females together) when all cSCC diagnoses in 1 year are registered instead of only the first tumor per patient. Together with our prediction analyses that showed an on average 26% further increase for the coming decade, this will have enormous implications for the dermato-oncological health care planning and cost management. Our results urgently call for revision of skin cancer health policies to be able to cope with this rising burden of cSCC management. Ultimately, primary prevention will remain the key strategy to halt the increasing trend in cSCC incidence and the occurrence of multiple cSCCs per patient, which we hope to further stimulate with our paper as well. MedicalResearch.com: What recommendations do you have for future research as a result of this work? Response: As we had data on multiple cSCCs for only one year, we would suggest to perform trend analyses for multiple cSCCs per patient as well when a longer follow-up duration has been reached. Furthermore, it would be very relevant to identify high-risk cSCCs or patients at risk for multiple cSCCs in order to be able to establish efficient follow-up regimens and take these high risk groups into account when revising skin cancer health policies. MedicalResearch.com: Is there anything else you would like to add? Response: The authors of this study have no conflicts of interest that are relevant to this article. Citation: Tokez S, Hollestein L, Louwman M, Nijsten T, Wakkee M. Incidence of Multiple vs First Cutaneous Squamous Cell Carcinoma on a Nationwide Scale and Estimation of Future Incidences of Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. Published online October 28, 2020. doi:10.1001/jamadermatol.2020.3677 [subscribe] Last Modified: [last-modified] The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.](https://medicalresearch.com/wp-content/uploads/2020/10/squamous-cell-cancer-dermnetnz-image.jpg)