Author Interviews, Infections, NIH, Ophthalmology / 05.12.2018
Prions of Creutzfeldt-Jakob Disease Detected Throughout Eye Tissues
MedicalResearch.com Interview with:
 Byron Caughey, Ph.D.
Senior Investigator
Chief, TSE/prion Biochemistry Section
Laboratory of Persistent Viral Diseases
NIH/NIAID Rocky Mountain Laboratories
Hamilton, MT 59840 USA
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Corneal transplants have caused the transmission of Creutzfeldt-Jakob disease (CJD) in at least two cases, and pathological prion protein has been detected in the retinas of the eyes of sporadic CJD cases. To build on these previous indications of prions in eye tissue, we tested the distribution of prions in various components of eyes from 11 sCJD decedents.
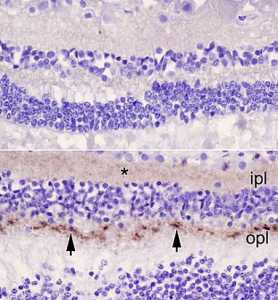
We applied a highly sensitive surrogate test for prions (RT-QuIC) that indicated that all of the sCJD cases had prions in multiple parts of their eye, including the cornea and sclera, which is the white outer surface of the eye. Retinas were usually contained the highest levels, in some cases approaching levels in the brain. Some other parts such as the cornea, lens and vitreous had much lower, but detectable, levels.
(more…)
Byron Caughey, Ph.D.
Senior Investigator
Chief, TSE/prion Biochemistry Section
Laboratory of Persistent Viral Diseases
NIH/NIAID Rocky Mountain Laboratories
Hamilton, MT 59840 USA
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Corneal transplants have caused the transmission of Creutzfeldt-Jakob disease (CJD) in at least two cases, and pathological prion protein has been detected in the retinas of the eyes of sporadic CJD cases. To build on these previous indications of prions in eye tissue, we tested the distribution of prions in various components of eyes from 11 sCJD decedents.
We applied a highly sensitive surrogate test for prions (RT-QuIC) that indicated that all of the sCJD cases had prions in multiple parts of their eye, including the cornea and sclera, which is the white outer surface of the eye. Retinas were usually contained the highest levels, in some cases approaching levels in the brain. Some other parts such as the cornea, lens and vitreous had much lower, but detectable, levels.
(more…)
 Byron Caughey, Ph.D.
Senior Investigator
Chief, TSE/prion Biochemistry Section
Laboratory of Persistent Viral Diseases
NIH/NIAID Rocky Mountain Laboratories
Hamilton, MT 59840 USA
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Corneal transplants have caused the transmission of Creutzfeldt-Jakob disease (CJD) in at least two cases, and pathological prion protein has been detected in the retinas of the eyes of sporadic CJD cases. To build on these previous indications of prions in eye tissue, we tested the distribution of prions in various components of eyes from 11 sCJD decedents.
We applied a highly sensitive surrogate test for prions (RT-QuIC) that indicated that all of the sCJD cases had prions in multiple parts of their eye, including the cornea and sclera, which is the white outer surface of the eye. Retinas were usually contained the highest levels, in some cases approaching levels in the brain. Some other parts such as the cornea, lens and vitreous had much lower, but detectable, levels.
(more…)
Byron Caughey, Ph.D.
Senior Investigator
Chief, TSE/prion Biochemistry Section
Laboratory of Persistent Viral Diseases
NIH/NIAID Rocky Mountain Laboratories
Hamilton, MT 59840 USA
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Corneal transplants have caused the transmission of Creutzfeldt-Jakob disease (CJD) in at least two cases, and pathological prion protein has been detected in the retinas of the eyes of sporadic CJD cases. To build on these previous indications of prions in eye tissue, we tested the distribution of prions in various components of eyes from 11 sCJD decedents.
We applied a highly sensitive surrogate test for prions (RT-QuIC) that indicated that all of the sCJD cases had prions in multiple parts of their eye, including the cornea and sclera, which is the white outer surface of the eye. Retinas were usually contained the highest levels, in some cases approaching levels in the brain. Some other parts such as the cornea, lens and vitreous had much lower, but detectable, levels.
(more…)