MedicalResearch.com Interview with:
Bernard Esquivel Zavala, MD, PhD, MHA
GenXys Chief Medical Officer
MedicalResearch.com: What is the mission of GenXys?
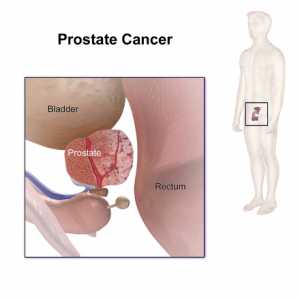
Response: Our mission at GenXys is to tailor the right treatment for each individual patient at the right time. GenXys founders, including Professors Pieter Cullis and Martin Dawes, were heavily involved in the precision medicine field from the very beginning, and they noticed a functional gap between the expectations and the actual clinical implementation of precision medicine Particularly, when it came to, at the time, the new field of pharmacogenetics. Their solution was to provide a comprehensive, user-friendly platform that organizes all patient data relevant to prescribing to provide the safest and most appropriate personalized prescribing options. Simply put, GenXys’ solutions were made by clinicians, for clinicians. The GenXys software suite collects patient information and categorizes that information, including pharmacogenetic data, based on clinical relevance and runs it through advanced condition -based algorithms to provide real time accurate prescribing options. It makes my life as a clinician easier and safer and gives me the confidence that I am not practicing ‘trial and error’ prescribing.
Ideally, every healthcare provider should be using a real time medication decision support solution like ours, and not just for pharmacogenetic test results. Pharmacogenomics is just one piece. In fact, our core product, TreatGx™ can run with or without pharmacogenomics. Let's say that you've run it without pharmacogenomics, meaning that you are using this tool to organize and rapidly identify how biophysical factors, liver function, kidney function, comorbidities, and drug-drug interactions may impact the medication you're about to prescribe to your patient. This functionality alone is incredibly helpful. In fact, the factors I just mentioned likely account for 95% of the reasons why a patient does not respond to a particular medication or might have an adverse drug reaction. But the TreatGx platform will also highlight when the evidence supports bringing pharmacogenomic information into the mix. The right approach is bringing all those relevant clinical, biochemical, and molecular factors closer to the provider which will ultimately foster personalization. We will start treating the individual instead of the disease(s).
As with any new technology, there are barriers to precision prescribing. This includes educational and emotional barriers. It’s important to educate providers and keep them up to date to help them understand the power that precision prescribing can bring into their practice—and the limitations—to set the right level of expectation. The Human Genome Project was finished in 2000, and there was a lot of buzz about pharmacogenomics even back in 2003. The field got a lot of traction in 2015. So, everyone thought, "Oh, this is going to be groundbreaking and quite disruptive. From now on my prescription is going to be a hundred percent accurate and safe." But it's not quite the whole story. Pharmacogenomics has to be considered as another piece of the puzzle. It's like saying that by having an MRI, you're curing cancer. It's just another piece of the treatment puzzle. There are also emotional barriers, where ego can factor into a decision. It can be uncomfortable for a physician to say, "I don't know this. Let me check it out. Let me explore it further, review, and come back to you." It's easier to say if I don't know it, that it doesn't work or isn’t relevant, rather than exposing yourself. And so that, in terms of the emotional piece, I would say is a big component. We can tackle the emotional component that element by fostering education and bringing education closer to providers.
(more…)


 Sean C. Rose, MD
Child Neurology
Nationwide Children’s Hospital
The Ohio State University, Columbus
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: There is conflicting evidence regarding the association between repetitive head impacts during youth contact sports and worse neurocognitive outcomes. Most research has been conducted in older adults, while the research in children is mostly limited to 1-2 sports seasons.
Sean C. Rose, MD
Child Neurology
Nationwide Children’s Hospital
The Ohio State University, Columbus
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: There is conflicting evidence regarding the association between repetitive head impacts during youth contact sports and worse neurocognitive outcomes. Most research has been conducted in older adults, while the research in children is mostly limited to 1-2 sports seasons.




 Shuchi Anand, MD MS (she/her)
Assistant Professor in Medicine
Director, Center for Tubulointerstitial Kidney Disease
Stanford University School of Medicine
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: A majority of people on dialysis who completed vaccination as of September 2021 have had a decline in antibody response to levels that would render them vulnerable to infection. Antibody response immediately after vaccination and circulating antibody response is strongly associated with risk for breakthrough after the initial vaccination series.
Shuchi Anand, MD MS (she/her)
Assistant Professor in Medicine
Director, Center for Tubulointerstitial Kidney Disease
Stanford University School of Medicine
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: A majority of people on dialysis who completed vaccination as of September 2021 have had a decline in antibody response to levels that would render them vulnerable to infection. Antibody response immediately after vaccination and circulating antibody response is strongly associated with risk for breakthrough after the initial vaccination series.