Decorative Alcohol Bottles May Contain Toxic Levels of Lead and Cadmium
 Dr Andrew Turner, PhD
Associate Professor (Reader) in Environmental Sciences
School of Geography, Earth and Environmental Sciences
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: This study was part of a wider study to look at potentially toxic metals in everyday household and consumer products.
The main findings here are that many enameled bottles, mainly used to store alcoholic beverages, contain very high levels of cadmium and lead in the décor. (more…)
Dr Andrew Turner, PhD
Associate Professor (Reader) in Environmental Sciences
School of Geography, Earth and Environmental Sciences
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: This study was part of a wider study to look at potentially toxic metals in everyday household and consumer products.
The main findings here are that many enameled bottles, mainly used to store alcoholic beverages, contain very high levels of cadmium and lead in the décor. (more…)High-Intensity Binge Drinking Linked to Abnormal Lipids and Liver Function Tests
Our study highlights the possible negative cardiovascular and hepatic impact associated with high-intensity binge drinking....
Americans Support Gene Therapies Even If They Cost More

MedicalResearch.com Interview with:
Wayne Winegarden, Ph.D.
Director, Center for Medical Economics and Innovation
Pacific Research Institute
MedicalResearch.com: What is the background for this poll? Would you tell us a little about the Center for Medical Economics and Innovation?
Response: Recent press reports have focused on how extensive innovative gene therapies can be. PRI was interested in learning where Americans stand on these cures of the future, and commission a new national opinion survey to find out.
The Center for Medical Economics and Innovation is a new center launched by PRI this spring to research and advance policies showing how a thriving biomedical and pharmaceutical sector benefits both patients and economic growth. Medical innovation is an important driver of economic growth, responsible for over $1.3 trillion in economic activity each year. As the Milken Institute has found, every job in the biomedical sphere supports another 3.3 jobs elsewhere in the economy.
Among the activities of the Center – which can be accessed at www.medecon.org – are providing free-market analysis to evaluate current policy proposals, producing easy-to-understand data and analysis on current trends in medical science, breaking down complex issues like pharmaceutical and biomedical pricing structures, and demonstrating the benefits that market-based reforms can offer patients and the U.S. health care system. (more…)
Atomwise Launches AI-Powered Virtual Drug Screening Program for Pediatric Cancers
 Abraham Heifets, PhD
Department of Computer Science
University of Toronto
MedicalResearch.com: What is the background for this announcement? How many children and adolescents are affected by pediatric cancer?
Response: Cancer is diagnosed in more than 15,000 children and adolescents each year. Many cancers, including pediatric cancer, do not have effective treatments and for those that do, it is estimated that 80% have serious adverse effects that impact long-term health.
(more…)
Abraham Heifets, PhD
Department of Computer Science
University of Toronto
MedicalResearch.com: What is the background for this announcement? How many children and adolescents are affected by pediatric cancer?
Response: Cancer is diagnosed in more than 15,000 children and adolescents each year. Many cancers, including pediatric cancer, do not have effective treatments and for those that do, it is estimated that 80% have serious adverse effects that impact long-term health.
(more…)Trends in Health Equity by Race/Ethnicity, Sex, and Income
If we want serious progress on health equity, we need serious research on its causes. ...
Limited Opioid Addiction Treatment Resources Should Be Geared Towards Most Affected Counties
Childhood Abuse More Likely With Male Caregiver, especially Mother’s Boyfriend
MedicalResearch.com Interview with:
Amanda Fingarson, DO Attending Physician, Child Abuse Pediatrics Ann and Robert H. Lurie Children’s Hospital of Chicago Assistant Professor of Pediatrics Feinberg Northwestern School of Medicine
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: Child physical abuse is a substantial pediatric public health issue, with significant morbidity and mortality. Studies have found that men, particularly children’s fathers and mothers’ boyfriends are common perpetrators of physical abuse. There is still a lack of knowledge, however, about the specific caregiver features that increase a child’s risk for physical abuse.
Our study design was unique, in that it was a multi-center study that compared young children with abusive and accidental injuries. Our primary finding was that abuse was much more likely when a male caregiver was present, and the resulting injuries were more likely to be severe or fatal. The presence of the mother’s boyfriend was the riskiest scenario, with the highest likelihood of abuse. Similarly, we found that caregiver relationships of less than 1 year increased the odds of abuse. Overall, the likelihood of abuse with female caregivers was much lower, with the exception of female babysitters. A final important finding of our study was that caregiving arrangements that were different than usual at the time of injury were at increased risk of abuse, suggesting that a stable and consistent caregiver is also important. (more…)
Spirometry Threshold Establishes Diagnosis of Clinically Significant COPD
Risk Prediction Nodule Helps Determine Cancer Risk of Pulmonary Nodule Found on CT Scan
Sexting Linked to Increased Sexual Activity and Substance Abuse Among Teenagers
 Camille Mori, B.A. (hons)
M.Sc. candidate
Clinical Psychology Program
Determinants of Child Development Lab
University of Calgary
MedicalResearch.com: What is the background for this study?
Response: Sexting, which is the sharing of sexual messages, images, or videos over technological devices, has recently become a cause for concern among parents, teachers, and policy makers. However, the research on sexting among youth is still in early stages, and evidence of the risks associated with sexting is inconsistent. One way to resolve discrepancies in the field is to conduct a meta-analysis, which statistically summarizes existing research. We conducted a meta-analysis in order to examine the association between sexting and sexual activity (having sex, multiple sexual partners, and lack of contraception use). The associations between sexting and mental health related variables, including delinquent behaviour, substance use, and depression/anxiety were also examined.
(more…)
Camille Mori, B.A. (hons)
M.Sc. candidate
Clinical Psychology Program
Determinants of Child Development Lab
University of Calgary
MedicalResearch.com: What is the background for this study?
Response: Sexting, which is the sharing of sexual messages, images, or videos over technological devices, has recently become a cause for concern among parents, teachers, and policy makers. However, the research on sexting among youth is still in early stages, and evidence of the risks associated with sexting is inconsistent. One way to resolve discrepancies in the field is to conduct a meta-analysis, which statistically summarizes existing research. We conducted a meta-analysis in order to examine the association between sexting and sexual activity (having sex, multiple sexual partners, and lack of contraception use). The associations between sexting and mental health related variables, including delinquent behaviour, substance use, and depression/anxiety were also examined.
(more…)Even With Reduced Permits, Hiking Yosemite’s Half Dome Just as Dangerous
Trailhead quotas are often used in national parks to limit the number of visitors and provide opportunities for solitude, but...
Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurologic Diseases
Acute Brain Injury: Detection of Brain Activation in Unresponsive Patients
Walgreens DisposeRx Program Will Provide Free Drug Disposal System at Pharmacies
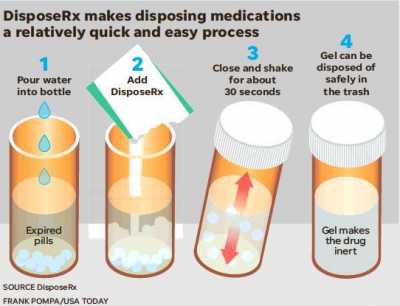 These consumers need to be educated about all the potential harm resulting from saving leftover medications. Leading pharmacy chains such as Walgreens are committed, as responsible corporate citizens, to making DisposeRx available upon request for their customers and to educate them about its use in getting rid of leftover drugs before they cause harm. Walgreens sees value in adding DisposeRx at-home solution to its comprehensive medication management and opioid stewardship programs as an additional method to reduce risks and exposure.
(more…)
These consumers need to be educated about all the potential harm resulting from saving leftover medications. Leading pharmacy chains such as Walgreens are committed, as responsible corporate citizens, to making DisposeRx available upon request for their customers and to educate them about its use in getting rid of leftover drugs before they cause harm. Walgreens sees value in adding DisposeRx at-home solution to its comprehensive medication management and opioid stewardship programs as an additional method to reduce risks and exposure.
(more…)Real World Study of Subcutaneous Abatacept (ORENCIA®) For Rheumatoid Arthritis
Statins May Double Risk of Diabetes
Individuals who used statins for the longest period of time (more than 2 years) had an even greater risk (3...
Drug Disposal Bags After Hospitalizations Can Get Rid of Some Leftover Opioids
Do Decision Aids Make a Difference in Prostate Cancer Screening?
We can learn about human food choice by studying reviews which capture how people feel about food and what they...
COLVERA™ Blood Test Provides Improved Effectiveness Detecting Recurrence of Colorectal Cancer
FARXIGA (dapagliflozin) Reduced Kidney Function Decline in Type II Diabetes
Does the HPV Vaccine Come With a Moral Hazard?
 Ali Moghtaderi PhD MBA
Assistant Research Professor and
Avi Dor PhD
Professor of Health Policy and Economics
Milken Institute School of Public Health
George Washington University
MedicalResearch.com: What is the background for this study?
Response: In this study, we investigate the effect of Human Papillomavirus (HPV) vaccination on participation in Pap test, which is one of the most effective cancer screening interventions. Cervical cancers are causally linked to HPV infections. The Pap test is a diagnostic procedure for early detection of cervical cancer. HPV vaccination provides partial protection against cervical cancer, and the Pap test is strongly recommended for women 21 to 65 years of age even after vaccination. If vaccination leads to a reduction in testing participation, it could contribute to greater incidence and severity of cervical cancer. Note that we focus on relatively older women (age 22 or older) who were not vaccinated at younger ages. (more…)
Ali Moghtaderi PhD MBA
Assistant Research Professor and
Avi Dor PhD
Professor of Health Policy and Economics
Milken Institute School of Public Health
George Washington University
MedicalResearch.com: What is the background for this study?
Response: In this study, we investigate the effect of Human Papillomavirus (HPV) vaccination on participation in Pap test, which is one of the most effective cancer screening interventions. Cervical cancers are causally linked to HPV infections. The Pap test is a diagnostic procedure for early detection of cervical cancer. HPV vaccination provides partial protection against cervical cancer, and the Pap test is strongly recommended for women 21 to 65 years of age even after vaccination. If vaccination leads to a reduction in testing participation, it could contribute to greater incidence and severity of cervical cancer. Note that we focus on relatively older women (age 22 or older) who were not vaccinated at younger ages. (more…)